EQUATE ANTI WRINKLE SPF 15
-
ensulizole and
octinoxate cream
Wal-Mart Stores Inc
----------
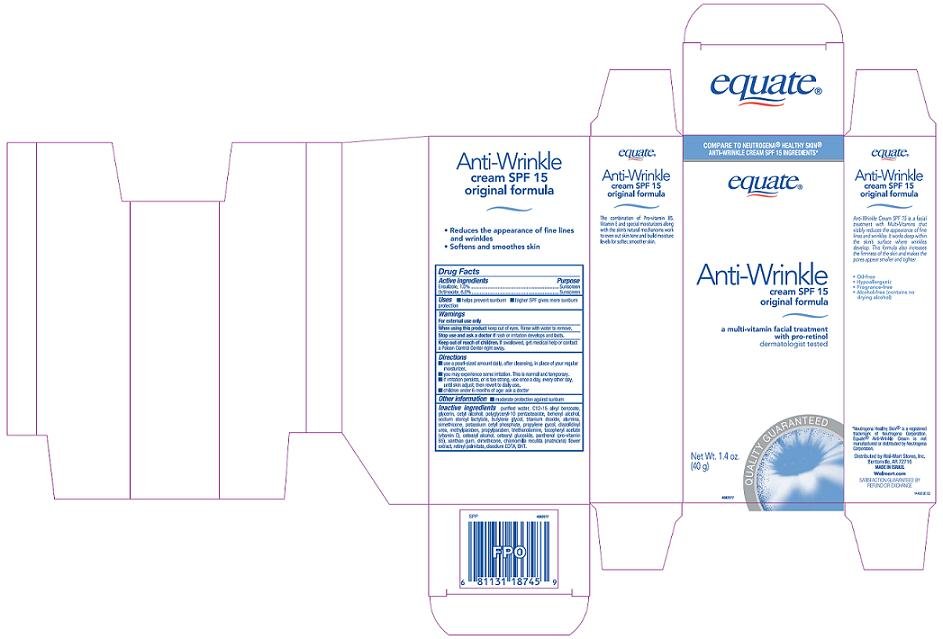
Wal-Mart Anti-Wrinkle Cream Drug FactsActive ingredient
Ensulizole, 1.0%
Octinoxate, 6.0%
Purpose
Sunscreen
Uses
- helps prevent sunburn
- higher SPF gives more sunburn protection
Warnings
For external use only
When using this product
keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
rash or irritation develops and lasts.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- use a pearl-sized amount daily, after cleansing, in place of your regular moisturizer.
- you may experience some irritation. This is normal and temporary.
- if irritation persists, or is too strong, use once a day, every other day, until skin adjusts: then revert to daily use.
- children under 6 months of age: ask a doctor
Other information
- moderate protection against sunburn
Inactive ingredients
purified water, C12-15 alkyl benzoate, glycerin, cetyl alcohol, polyglyceryl-10 pentastearate, behenyl alcohol, sodium steroyl lactylate, butylene glycol, titanium dioxide, alumina, simethicone, potassium cetyl phosphate, propylene glycol, diazolidinyl urea, methylparaben, propylparaben, triethanolamine, tocopheryl acetate (vitamin E), cetearyl alcohol, cetearyl glucoside, panthenol (pro-vitamin B5), xanthan gum, dimethicone, chamomilla recutita (matricaria) flower extract, retinyl palmitate, disodium EDTA, BHT
Principal Display Panel
Compare to Neutrogena® Healthy Skin® Anti-Wrinkle Cream SPF 15 ingredients
Anti-Wrinkle Cream SPF 15
Original Formula
A multi-vitamin facial treatment with pro-retinol
Dermatologist Tested
Anti-Wrinkle Cream Carton
| EQUATE ANTI WRINKLE
SPF 15
ensulizole, octinoxate cream |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part352 | 05/07/2009 | |
| Labeler - Wal-Mart Stores Inc (051957769) |