ACCUHIST DROPS
-
phenylephrine hydrochloride and
chlorpheniramine maleate solution
Tiber Laboratories LLC
----------
Accuhist Drops - Chlorpheniramine Maleate and Phenylephrine Hydrochloride SolutionOTC - ACTIVE INGREDIENT
Phenylephrine Hydrochloride 2.5 mg/1mL: decongestant; Chlorpheniramine Maleate 1.0 mg/1mL: antihistamine
OTC - PURPOSE
Temporarily relieves: runny nose; reduces sneezing; itching of the nose or throat; itchy, watery eyes due to hay fever or other upper respiratory allergies; nasal congestion due to the common cold; temporarily restores free breathing through the nose
WARNINGS
May cause excitability especially in children. May cause drowsiness. Sedatives and tranquilizers may increase drowsiness effect. Do not give this product to children who are taking sedatives or tranquilizers, without first consulting the child’s doctor. Do not exceed recommended dosage. If nervousness, dizziness, or sleeplessness occur, discontinue use and consult a doctor. If symptoms do not improve within 7 days or are accompanied by fever, consult a doctor. Do not give this product to a child who has heart disease, high blood pressure, thyroid disease, or diabetes unless directed by a doctor. Do not use in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child’s prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product. Do not give this product to children who have a breathing problem such as chronic bronchitis, or who have glaucoma, without first consulting the child’s doctor.
USAGE
2-6 years: = (1 mL) every 4 to 6 hours
6-12 years: = (2 mL) every 4 to 6 hours
Do not exceed 4 doses during a 24-hour period or as directed by a doctor.
Keep this and all drugs out of the reach of children.
Other Information: Store at controlled room temperature 20° – 25°C (68° – 77°F)
INACTIVE INGREDIENT
Cherry Flavor, Citric Acid USP, Glycerin USP, Purified Water, Sodium Benzoate USP, 70% Sorbitol Solution USP.
Questions? Call 678-208-0388 24 hours a day, 7 days a week.
Under Federal Law, AccuHist Drops are available without a prescription. Certain laws may differ.
Manufactured for:
Tiber Laboratories, LLC.
Suwanee, GA 30024
Rev. 10/09 030-21 P0359
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

Figure 1. Cover and Base of Package Label
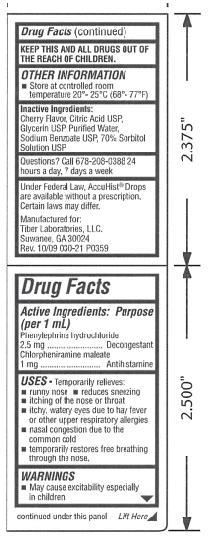
Figure 2. Page 4 and Cover of Package Label
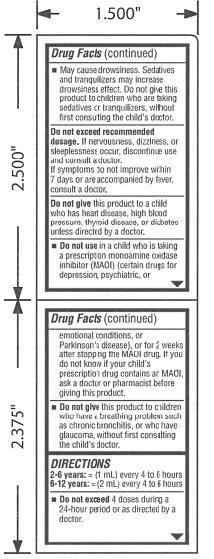
Figure 3. Page 2 and Page 3 of Package Label
| ACCUHIST DROPS
2.5 mg phenylephrine hydrochloride, 1mg chrlorpheniramine maleate per 1 ml oral solution solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part341 | 01/25/2010 | |
| Labeler - Tiber Laboratories LLC (008913939) |
| Registrant - Gorbec Pharmaceutical Services Inc. (791919678) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Gorbec Pharmaceutical Services Inc. | 791919678 | MANUFACTURE | |