ENEMA
-
mineral oil enema
Amerisource Bergen Drug Corporation
----------
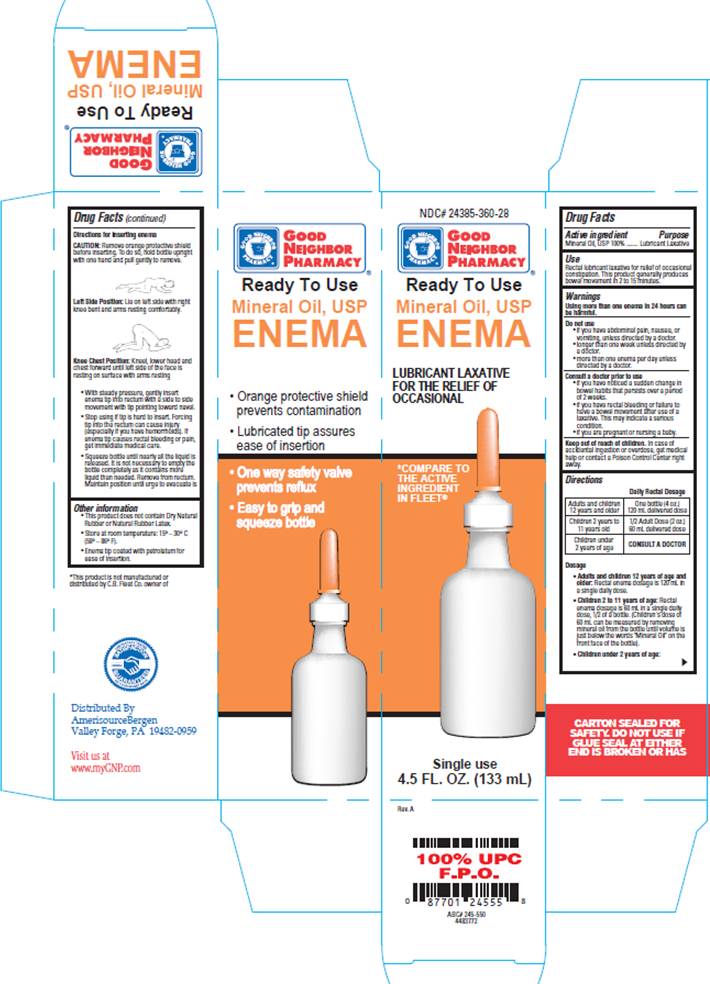
DRUG FACTSACTIVE INGREDIENT
Mineral Oil, 100%
PURPOSE
Lubricant Laxative
USE
- For relief of occasional constipation. This product generally produced bowel movement in 2 to 15 minutes.
WARNINGS
Using more than one enema in 24 hours can be harmful.
Do not use
- If you have abdominal pain, nausea, or vomiting, unless directed by a doctor.
- Longer than one week unless directed by a doctor.
- More than one enema per day unless directed by a doctor.
Consult a doctor before use
- if you have noticed a sudden change in bowel habits that persist over a period of 2 weeks
- if you have rectal bleeding or failure to have a bowel movement after use of a laxative. This may indicate a serious condition.
- if you are pregnant or nursing a baby
Keep out of reach of children
In case of accidental injestion or overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS
Daily Rectal Dosage| Adults and children 12 years and older | One bottle (4 oz.) 120 mL delivered dose |
| Children 2 years to 11 years old | 1/2 Adult dose (2 oz.) 60 mL delivered dose |
| Children under 2 years of age | CONSULT A DOCTOR |
Dosage
-
Adults and children 12 years of age and older: rectal enema dosage is 120 milliliters in a single daily dose.
-
Children 2 to 11 years of age: rectal enema dosage is 60 mililiters in a single daily dose, 1/2 of a bottle. (Children's dose of 60 mililiters can be measured by removing mineral oil from the bottle until volume is just under the words "Mineral Oil" on the front face of the bottle).
-
Children under 2 years of age:
Consult a doctor.
CAUTION:Remove orange protective shield before inserting. To do so, hold bottle upright with one hand and pull gently to remove.
Positioning:
Left side position: Lie on left side with right knee bent and arms resting comfortably.
Knee chest position: Kneel, lower head and chest forward until left side of the face is resting on surface with arms resting comfortably.
- With steady pressure, gently insert enema tip into rectum with a side to side movement with tip pointing toward navel.
- Stop using if tip is hard to insert. Forcing tip into the rectum can cause injury (especially if you have hemorrhoids). If enema tip causes rectal bleeding or pain, get immediate medical care.
- Squeeze bottle until nearly all the liquid is released. Remove from rectum. Maintain position until urge to evacuate is strong (usually 2 to 15 minutes).
OTHER INFORMATION
- This product does not contain Dry Natural Rubber or Natural Rubber Latex.
- Store at room temperature 15° - 30° C (59° - 86° F)
- Enema tip coated with petrolatum for ease of insertion
PACKAGE INFORMATION
NDC# 24385-360-28GOOD NEIGHBOR PHARMACY®
Ready to Use
Mineral Oil, USP
ENEMA
LUBRICANT LAXATIVE FOR THE RELIEF OF OCCASIONAL CONSTIPATION
* COMPARE TO THE ACTIVE INGREDIENT IN FLEET®
Single use
4.5 FL. OZ. (133 ml)
- Orange protective shield prevents contamination
- Lubricated tip assures ease of insertion
- One way safety valve prevents reflux
- Easy to grip and squeeze bottle
*This product is not manufactured or distributed by C.B. Fleet Co., owner of the registered trademark Fleet®.
Distributed by
Amerisource Bergen
Valley Forge, PA 19482-0959
| ENEMA
mineral oil enema |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part334 | 02/13/2004 | 03/01/2010 |
| Labeler - Amerisource Bergen Drug Corporation (007914906) |