HEMORRHOIDAL STARCH
-
starch, corn suppository
Walgreen Company
----------
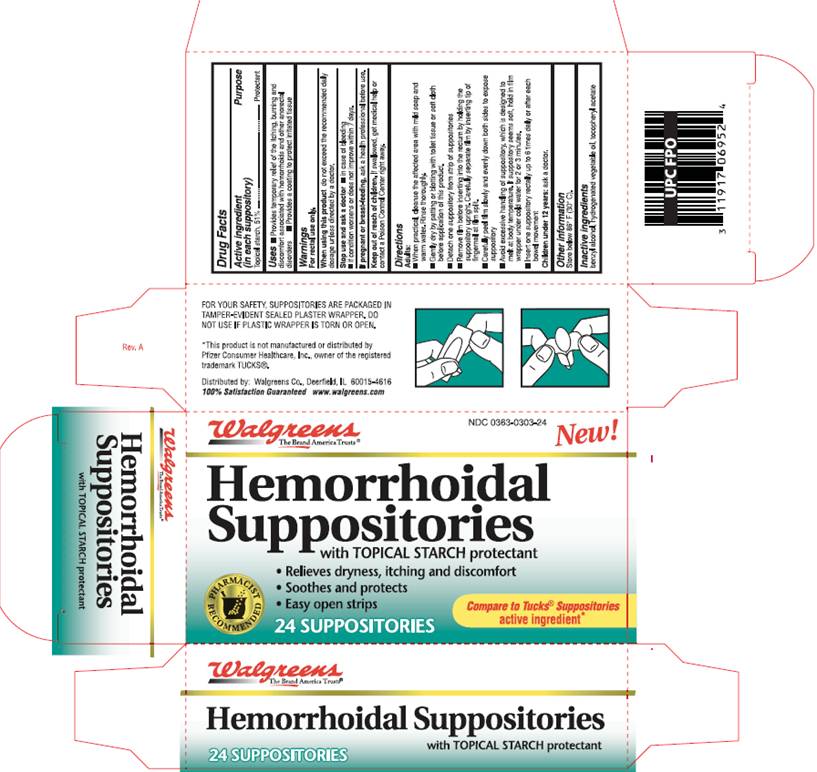
DRUG FACTSACTIVE INGREDIENT
Topical starch, 51%
PURPOSE
Protectant
USE
- Provides temporary relief of the itching, burning and discomfort associated with hemorrhoids and other anorectal disorders
- Provides a coating to protect irritated tissue
WARNINGS
For rectal use only.
When using this product
do not exceed the recommended daily dosage unless directed by a doctor.
Stop use and ask a doctor
- in case of bleeding
- if condition worsens or does not improve within 7 days.
If pregnant or breast-feeding,
ask a health professional before use.Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
Adults:- When practical, cleanse the affected area with mild soap and warm water. Rinse thoroughly.
- Gently dry by patting or blotting with toilet tissue or soft cloth before application of this product.
- Detach one suppository from strip of suppositories.
- Remove film before inserting into the rectum by holding the suppository upright. Carefully separate film by inserting tip of fingernail at film split.
- Carefully peel film slowly and evenly down both sides to expose suppository
- Avoid excessive handling of suppository, which is designed to melt at body temperature. If suppository seems soft, hold in film wrapper under cold water for 2 or 3 minutes.
- Insert one suppository rectally up to 6 times daily or after each bowel movement
OTHER INFORMATION
Store below 86° F (30° C).INACTIVE INGREDIENTS
benzyl alcohol, hydrogenated vegetable oil, tocopheryl acetate
PACKAGE INFORMATION
WalgreensThe Brand America Trusts®
NDC 0363-0303-24
NEW!
Hemorrhoidal
Suppositories
with TOPICAL STARCH protectant
- relieves dryness, itching and discomfort
- Soothes and protects
- Easy open strips
compare to Tucks® Suppositories
active ingredient*
FOR YOUR SAFETY, SUPPOSITORIES ARE PACKAGED IN
TAMPER-EVIDENT SEALED PLASTIC WRAPPER. DO
NOT USE IF PLASTIC WRAPPER IS TORN OR OPEN.
*This product is not manufactured or distributed by Pfizer consumer Healthcare, Inc., owner of the registered trademark TUCKS®.
Distributed by: Walgreens Co., Deerfield, IL 60015-4616
100% Satisfaction Guaranteed www.walgreens.com
| HEMORRHOIDAL STARCH
starch suppository |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part346 | 12/27/2005 | 11/30/2009 |
| Labeler - Walgreen Company (008965063) |