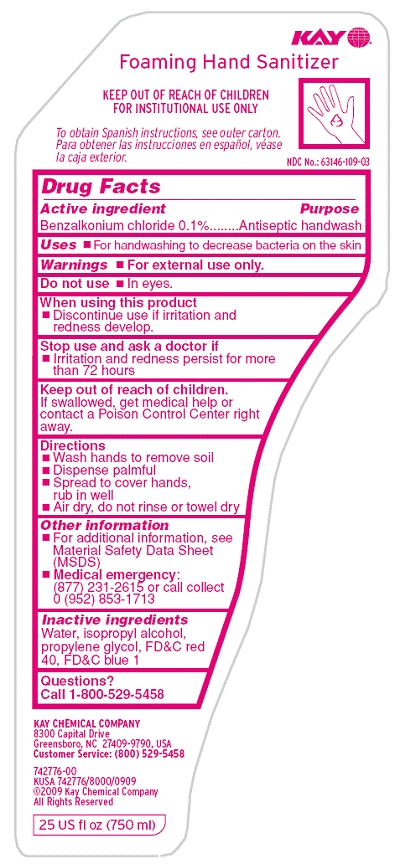
KAY FOAMING HAND SANITIZER
-
benzalkonium chloride solution
Kay Chemical Company
----------
Drug FactsActive ingredient
Benzalkonium chloride 0.1%
Purpose
Antiseptic handwash
Warnings
-
For external use only.
Do not use
- In eyes.
When using this product
- Discontinue us if irritation and redness develop.
Stop use and ask a doctor if
- Irritation and redness persist for more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Wash hands to remove soil
- Dispense palmful
- Spread to cover hands, rub in well
- Air dry, do not rinse or towel dry
Other information
- For additional information, see Material Safety Data Sheet (MSDS)
-
Medical Emergency: (877) 231-2615 or call collect 0 (952) 853-1713
Inactive ingredients
Water, isopropyl alcohol, propylene glycol, FDC red 40, FDC blue 1
Questions?
Call 1-800-529-5458
Principal Display Panel/Representative Label
Kay
Foaming Hand Sanitizer
KEEP OUT OF REACH OF CHILDREN
FOR INSTITUTIONAL USE ONLY
To obtain Spanish instructions, see outer carton.
NDC No.: 63146-109-03
Kay Chemical Company
8300 Capital Drive
Greensboro, NC 27409-9790, USA
Customer Service: (800) 529-5458
742776-00
KUSA 742776/8000/0909
(C)2009 Kay Chemical Company
All rights reserved
25 US fl oz (750 ml)
| KAY FOAMING HAND SANITIZER
antiseptic handwash solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part333 | 02/22/2008 | |
| Labeler - Kay Chemical Company (003237021) |