ALAHIST DM
-
brompheniramine maleate,
phenylephrine hydrochloride and
dextromethorphan hydrobromide liquid
Poly Pharmaceuticals, Inc.
----------
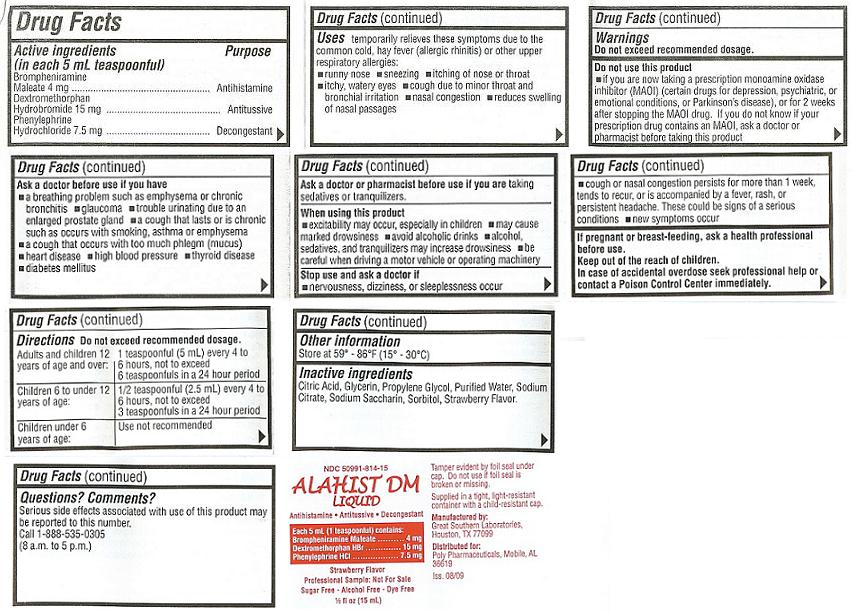
ALAHIST DM LIQUIDDrug Facts
Active ingredients Purpose
(in each 5 mL teaspoonful)
Brompheniramine Maleate 4 mg ............ Antihistamine
Dextromethorphan Hydrobromide 15 mg..... Antitussive
Phenylephrine Hydrochloride 7.5mg....... Decongestant
Uses
temporarily relieves these symptoms due to the common cold, hay fever (allergic rhinitis) or other upper respiratory allergies:
- runny nose
- sneezing
- itching of nose or throat
- itchy, watery eyes
- cough due to minor throat and bronchial irritation
- nasal congestion
- reduces swelling of nasal passages
Warnings
Do not exceed recommended dosage.
Do not use this product
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
- a cough that lasts or is chronic such as occurs with smoking, asthma or emphysema
- a cough that occurs with too much phlegm (mucus)
- heart disease
- high blood pressure
- thyroid disease
- diabetes mellitus
Ask a doctor or pharmacist before use if you are
taking sedatives or tranquilizers.
When using this product
- excitability may occur, especially in children
- may cause marked drowsiness
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur
- cough or nasal congestion persists for more than 1 week, tends to recur, or is accompanied by a fever, rash, or persistent headache. These could be signs of a serious condition.
- new symptoms occur
If pregnant or breast-feeding,
ask a health professional before use.Keep out of the reach of children.
In case of accidental overdose seek professional help or contact a Poison Control Center immediately.Directions
Do not exceed recommended dosage.| Adults and children 12 years of age and over: | 1 teaspoonful (5 mL) every 4 to 6 hours, not to exceed 6 teaspoonfuls in a 24 hour period |
| Children 6 to under 12 years of age: | 1/2 teaspoonful (2.5 mL) every 4 to 6 hours, not to exceed 3 teaspoonfuls in a 24 hour period |
| Children under 6 years of age: | Use not recommended |
Other information
Store at 59o - 86oF (15o-30oC)Inactive ingredients
Citric Acid, Glycerin, Propylene Glycol, Purified Water, Sodium Citrate, Sodium Saccharin, Sorbitol, Strawberry Flavor.Questions? Comments?
Serious side effects associated with use of this product may be reported to this number.Call 1-800-882-1041
Mon.-Fri. (8 a.m. to 5 p.m. CST).
PRODUCT PACKAGING
The packaging below represents the labeling currently used:
Principal Display Panel and Side Panel for 473mL Label:
NDC 50991-814-16
ALAHIST DM LIQUID
Antihistamine / Antitussive
Decongestant
Each 5 mL (1 teaspoonful) contains:
Brompheniramine Maleate........... 4 mg
Dextromethorphan HBr............... 15 mg
Phenylephrine HCl.................... 7.5 mg
Strawberry Flavor
SUGAR FREE / ALCOHOL FREE
DYE FREE
Distributed by:
Poly Pharmaceuticals
Mobile, AL 36619
16 fl oz. (473 mL)
Tamper evident by foil seal under cap. Do not use if foil seal is broken or missing.
Dispense in a tight, light-resistant container with a child-resistant cap.
This bottle is not to be dispensed to consumer.
Manufactured by: Great Southern Laboratories, Houston, TX 77099
Distributed by: Poly Pharmaceuticals, Mobile, AL 36619
Iss. 11/09
Principal Display Panel and Side Panel for 15 mL Label:
NDC 50991-814-15
ALAHIST DM LIQUID
Antihistamine / Antitussive / Decongestant
Each 5 mL (1 teaspoonful) contains:
Brompheniramine Maleate ............ 4 mg
Dextromethorphan HBr................ 15 mg
Phenylephrine HCl......................7.5 mg
Strawberry Flavor
Professional Sample: Not For Sale
Sugar Free / Alcohol Free / Dye Free
1/2 fl oz (15 mL)
Tamper evident by foil seal under cap. Do not use if foil seal is broken or missing.
Supplied in a tight, light-resistant container with a child-resistant cap.
Manufactured by:
Great Southern Laboratories
Houston, TX 77099
Distributed for:
Poly Pharmaceuticals, Mobile AL
36619
Iss. 11/09




| ALAHIST
DM
brompheniramine maleate, phenylephrine hydrochloride, dextromethorphan hydrobromide liquid |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part341 | 12/27/2007 | |
| Labeler - Poly Pharmaceuticals, Inc. (198449894) |
| Registrant - Great Southern Laboratories (056139553) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Great Southern Laboratories | 056139553 | manufacture | |