J-TAN PD
-
brompheniramine maleate liquid
JayMac Pharmaceuticals LLC
----------
J-TAN PD DropsJ-Tan PD DROPS
NDC 64661-031-30Rx Only
DESCRIPTION
J-Tan PD is an antihistaminic drop for oral administration containing the following amount of active
ingredient in each 1 mL dropperful:
Brompheniramine Maleate, USP............... 1 mg
Inactive ingredients: Glycerin, Sorbitol, Propylene Glycol, Citric Acid, Sodium Citrate, Sodium Saccharin,
Banana Flavor, Strawberry Flavor, Purified Water.
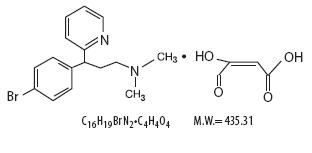
Brompheniramine maleate is an antihistamine having the chemical name, 2-pyridinepropanamine,
γ-(4-bromophenyl)-N, N-dimethyl-, (±)-, (Z)-2-butendioate (1:1), with the following chemical structure:
CLINICAL PHARMACOLOGY
Brompheniramine MaleateBrompheniramine maleate is a histamine antagonist, specifically an H1-receptor-blocking agent belonging
to the alkylamine class of antihistamines. Antihistmaines appear to compete with histamine for receptor sites
on effector cells. Brompheniramine also has anticholinergic (drying) and sedative effects. Among the
antihistaminic effects, it antagonizes the allergic response (vasodilatation, increased vascular permeability,
increased mucus secretion) of nasal tissue. Brompheniramine is well absorbed from the gastrointestinal
tract, with peak plasma concentration after a single, oral dose of 4 mg reached in 5 hours; urinary excretion
is the major route of elimination, mostly as products of biodegradation; the liver is assumed to be the main
site of metabolic transformation.
INDICATIONS AND USAGE
J-Tan PD Drops is indicated for symptomatic relief of upper respiratory symptoms, including nasal congestion,associated with allergy or the common cold.
CONTRAINDICATIONS
Patients with hypersensitivity or idiosyncrasy to Brompheniramine Maleate. Do not use in newborn infants,premature infants, in nursing mothers, in patients with severe hypertension, severe coronary artery disease,
ischemic heart disease, or in those receiving monoamine oxidase (MAO) inhibitors. Antihistamines are
contraindicated in patients with narrow-angle glaucoma, urinary retention, peptic ulcer, and during as asthma
attack. Antihistamines should not be used to treat lower respiratory tract conditions including asthma.
WARNINGS
Antihistamines may diminish mental alertness, and may cause hyperexcitability especially in children.At doses higher than the recommended dose, nervousness, dizziness, or sleeplessness may occur.
Especially in infants and small children, antihistamines in overdosage may cause hallucinations,
convulsions, and death. Use with caution in patients with hypertension, ischemic heart disease, and
persons older than 60 years old. Do not give this product to children who have a chronic pulmonary
disease, breathing problems such as chronic bronchitis, glaucoma, or those who are taking sedatives
or tranquilizers without first consulting with a doctor. Considerable caution should be exercised in
patients with hypertension, diabetes mellitus, ischemic heart disease, hyperthyroidism, increased
intraocular pressure and prostatic hypertrophy.
Do not exceed the recommended dosage. If nervousness, dizziness, or sleeplessness occurs,
discontinue use and consult a doctor.
PRECAUTIONS
GeneralBecause J-Tan PD Drops contain an antihistamine, it should be used with caution in patients with a history
of bronchial asthma, narrow angle glaucoma, gastrointestinal obstruction, or urinary bladder neck obstruction.
Due to its sympathomimetic component, J-Tan PD Drops should be used with caution in patients with
diabetes, hypertension, heart disease, or thyroid disease.
Information for Patients (or Parents)
Patients (or parents) should be warned about engaging in activities requiring mental alertness. In mild cases
or in particularly sensitive patients, less frequent doses may be adequate.
Drug Interactions:
Do not use this medication in a child who is taking a prescription monoamine oxidase (MAO) inhibitor (certain
drugs used for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after
stopping the MAO inhibitor. MAO inhibitors prolong and intensify the anticholinergic effects of antihistamines
and may enhance the effect of pseudoephedrine HCl. Sympathomimetic agents may reduce the effects of
anti-hypertensive drugs. Antihistamines have additive effects with alcohol and other CNS (Central Nervous
System) depressants (hypnotics, sedatives, tranquilizers, anti-anxiety agents, etc.).
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies have not been performed to assess the carcinogenic and mutagenic potential of J-Tan PD Drops
or its effect on fertility.
Pregnancy, Teratogenic Effects - Pregnancy Category C
Animal reproductive studies have not been performed with J-Tan PD Drops. It is also not known if it can
cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. J-Tan PD
Drops should be given to a pregnant woman only if clearly needed.
Nursing Mothers
Because of the higher risk of intolerance of antihistamines in small infants generally, and in newborns and
prematures in particular, J-Tan PD Drops is contraindicated in nursing mothers.
Geriatric Use
The elderly (60 year of age or older) are more likely to experience adverse reactions. Caution should be
taken when prescribing this drug to the elderly.
ADVERSE REACTIONS
The most frequent adverse reactions to J-Tan PD Drops include: sedation, dryness of mouth, nose, andthroat; thickening of bronchial secretions; dizziness.
Other adverse reactions may include:
Dermatologic - urticaria, drug rash, photosensitivity, pruritis.
Cardiovascular System - hypotension, hypertension, cardiac arrhythmias, palpitations.
Central Nervous System (CNS) - disturbed coordination, tremor, irritability, insomnia, visual
disturbances, weakness, nervousness, convulsion, headache, euphoria, and dysphoria.
G.U. System - urinary frequency, difficult urination.
G.I. System - epigastric discomfort, anorexia, nausea, vomiting, diarrhea, constipation.
Respiratory System - tightness of chest and wheezing, shortness of breath.
Hematologic System - hemolytic anemia, thrombocytopenia, agranulocytosis.
OVERDOSAGE
Signs and SymptomsCentral nervous system effects from overdosage of brompheniramine may vary from depression to
stimulation, especially in children.
Anticholinergic effects may also occur.
Treatment
Include emesis if patient is alert and is seen prior to 6 hours following ingestion. Precautions against aspiration
must be taken, especially in infants and small children. Gastric lavage may be carried out, but in some cases
tracheotomy may be needed prior to lavage. For CNS hyperactivity or convulsive seizures, intravenous
shortacting barbiturates may be indicated. Hypertensive responses and/or tachycardia should be treated
appropriately. Oxygen, intravenous fluids and other supportive measures should be used as indicated.
DOSAGE AND ADMINISTRATION:
Take by mouth only.| AGE | DOSE* | FREQUENCY |
| 6 to under 12 years of age | 2 dropperfuls (2 mL) | 4 times per day |
*In mild cases or in particularly sensitive patients, less frequent or reduced doses may be adequate.
HOW SUPPLIED
J-Tan PD Drops is a strawberry-banana-flavored, sugar free, alcohol free, dye free liquid packaged in 30 mLbottles for dropper dosage, NDC 64661-031-30. Calibrated, shatterproof dropper enclosed in each carton.
STORAGE
Store at 20o-25oC (68o-77oF). Dispense in a tight, light-resistant container as defined in the USP/NFwith a child-resistant cap. Dispense in the original container. Avoid exposure to excessive heat.
Keep tightly closed.
Tamper evident by foil seal under cap. Do not use if foil seal is broken or missing.
WARNING: KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
IN CASE OF ACCIDENTAL OVERDOSE, SEEK PROFESSIONAL ASSISTANCE OR
CONTACT A POISON CONTROL CENTER IMMEDIATELY.
Rx Only
Manufactured by:
Great Southern Laboratories
Houston, TX 77099
Manufactured for:
JayMac Pharmaceuticals
Sunset, LA 70584
Rev. 02/08
PRODUCT PACKAGING:
The packaging below represents the labeling used:Principal Display Panel and Side Panels for 30 mL Label:
NDC 64661-031-30
J-TAN PD
Drops
Each dropperful (1 mL) contains:
Brompheniramine Maleate.... 1 mg
ANTIHISTAMINE
SUGAR FREE / ALCOHOL FREE
DYE FREE
STRAWBERRY-BANANA FLAVOR
Rx Only
1 fl oz (30 mL)
USUAL DOSAGE*:
Children 6 to under 12 years of age - 2 dropperfuls (2.0 mL).
Not to exceed 4 doses in a 24 hour period.
*In mild cases or in particularly sensitive patients, less frequent or reduced doses may be adequate.
For complete information on use, see attached insert.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. IN CASE OF
ACCIDENTAL OVERDOSE, SEEK PROFESSIONAL ASSISTANCE OR CONTACT A
POISON CONTROL CENTER IMMEDIATELY.
STORAGE: Store between 20o-25oC (68o-77oF). Avoid exposure to excessive heat. Keep tightly closed.
Tamper evident by foil seal under cap. Do not use if foil seal is broken or missing.
Manufactured by:
Great Southern Laboratories
Houston, TX 77099
Manufactured for:
JayMac Pharmaceuticals
Sunset, LA 70584
Rev. 02/08


| J-TAN
PD
brompheniramine maleate liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part341 | 05/29/2007 | |
| Labeler - JayMac Pharmaceuticals LLC (830767260) |
| Registrant - Great Southern Laboratories (056139553) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Great Southern Laboratories | 056139553 | manufacture | |