CHLO TUSS
-
chlophedianol hydrochloride,
dexbrompheniramine maleate and
phenylephrine hydrochloride liquid
R.A. McNeil Company
----------
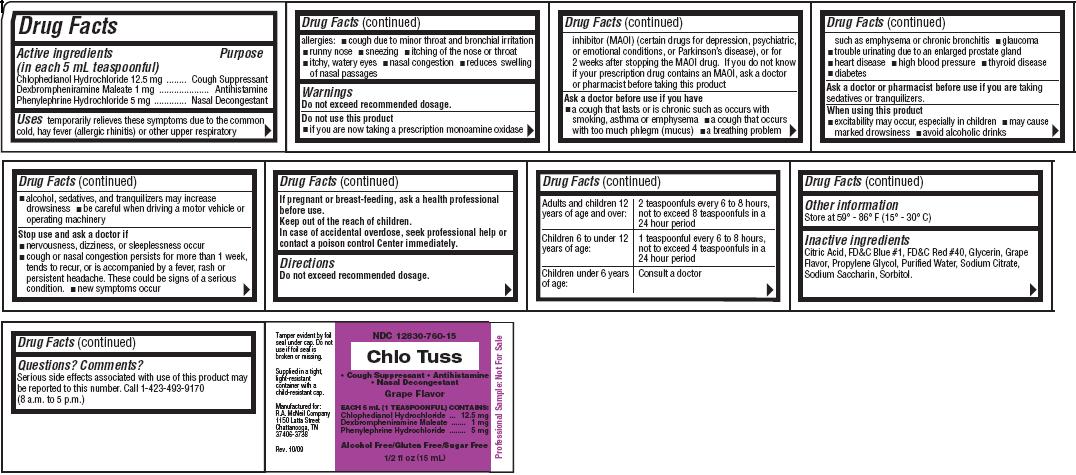
Chlo TussDrug Facts
Active ingredients Purpose
(in each 5 mL teaspoonful)
Chlophedianol Hydrochloride 12.5 mg .... Cough Suppressant
Dexbrompheniramine Maleate 1 mg..................Antihistamine
Phenylephrine Hydrochloride 5 mg ........ Nasal Decongestant
Uses
temporarily relieves these symptoms due to the common cold, hay fever (allergic rhinitis) or other upper respiratory allergies:
- cough due to minor throat and bronchial irritation
- runny nose
- sneezing
- itching of the nose or throat
- itchy, watery eyes
- nasal congestion
- reduces swelling of nasal passages
Warnings
Do not exceed recommended dosage.
Do not use this product
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product
Ask a doctor before use if you have
- a cough that lasts or is chronic such as occurs with smoking, asthma or emphysema
- a cough that occurs with too much phlegm (mucus)
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
- heart disease
- high blood pressure
- thyroid disease
- diabetes
Ask a doctor or pharmacist before use if you are
taking sedatives or tranquilizers.
When using this product
- excitability may occur, especially in children
- may cause marked drowsiness
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur
- cough or nasal congestion persists for more than 1 week, tends to recur, or is accompanied by a fever, rash or persistent headache. These could be signs of a serious condition.
- new symptoms occur
If pregnant or breast-feeding,
ask a health professional before use.Keep out of the reach of children.
In case of accidental overdose, seek professional help or contact a poison control center immediately.Directions
Do not exceed recommended dosage.
| Adults and children 12 years of age and over: | 2 teaspoonfuls every 6 to 8 hours, not to exceed 8 teaspoonfuls in a 24 hour period |
| Children 6 to under 12 years of age: | 1 teaspoonful every 6 to 8 hours, not to exceed 4 teaspoonfuls in a 24 hour period |
| Children under 6 years of age: | Consult a doctor |
Other information
Store at 59o-86oF (15o-30oC)
Inactive ingredients
Citric Acid, FD and C Blue #1, FD and C Red #40, Glycerin, Grape Flavor, Propylene Glycol, Purified Water, Sodium Citrate, Sodium Saccharin, Sorbitol.
Questions? Comments?
Serious side effects associated with use of this product may be reported to this number. Call 1-423-493-9170
(8 a.m. to 5 p.m.)
PRODUCT PACKAGING
The packaging below represents the labeling currently used:
Principal Display Panel and Side Panel for 473mL Label:
NDC 12830-760-16
Chlo Tuss
- Cough Suppressant
- Antihistamine
- Nasal Decongestant
EACH 5 mL (1 TEASPOONFUL) CONTAINS:
Chlophedianol Hydrochloride........... 12.5 mg
Dexbrompheniramine Maleate............ 1 mg
Phenylephrine Hydrochloride.............. 5 mg
Alcohol Free / Gluten Free
Sugar Free
ONE PINT (473 mL)
Mfg. for R.A. McNeil Company
Chattanooga, TN 37406-3738
Rev. 09/09
Tamper evident by foil seal under cap. Do not use if foil seal is broken or missing.
Dispense in a tight, light-resistant container with a child-resistant cap.
This package not intended for dispensing to the patient.
Manufactured for:
R.A. McNeil Company
1150 Latta Street
Chattanooga, TN 37406-3738
Rev. 09/09
Principal Display Panel and Side Panel for 15 mL Label:
NDC 12830-760-15
Chlo Tuss
- Cough Suppressant
- Antihistamine
- Nasal Decongestant
EACH 5 mL (1 TEASPOONFUL) CONTAINS:
Chlophedianol Hydrochloride.... 12.5 mg
Dexbrompheniramine Maleate ..... 1 mg
Phenylephrine Hydrochloride........ 5 mg
Alcohol Free/Gluten Free/Sugar Free
1/2 fl oz (15 mL)
Tamper evident by foil seal under cap. Do not use if foil seal is broken or missing.
Supplied in a tight, light-resistant container with a child-resistant cap.
Manufactured for:
R.A. McNeil Company
1150 Latta Street
Chattanooga, TN 37406-3738
Rev. 10/09
Professional Sample: Not For Sale



| CHLO TUSS
chlophedianol hydrochloride, dexbrompheniramine maleate, phenylephrine hydrochloride liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part341 | 10/27/2009 | |
| Labeler - R.A. McNeil Company (008305220) |