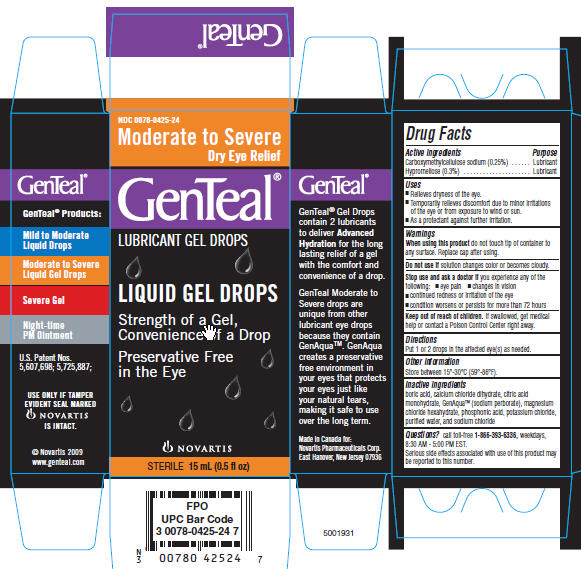
GENTEAL MODERATE TO SEVERE GEL DROPS
-
hypromellose and
carboxymethylcellulose sodium gel
Novartis Pharmaceuticals Corporation
----------
Drug FactsActive ingredient
Carboxymethylcellulose sodium (0.25%)
Hypromellose (0.3%)
Purpose
Lubricant
Uses
- Relieves dryness of the eyes.
- Temporarily relieves discomfort due to the minor irritation of the eye or from exposure to wind or sun.
- As a protectant against further irritation.
Warnings
When using this product do not touch tip of container to any surface. Replace cap after using.
Do not use if solution changes color or becomes cloudy.
Stop use and ask a doctor if you experience any of the following:
- eye pain
- changes in vision
- continued redness or irritation of the eye
- condition worsens or persists more than 72 hours
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Put 1 or 2 drops in the affected eye(s) as needed.
Other Information
Store between 15⁰- 30⁰C (59⁰-86⁰F).
Inactive ingredients
Boric acid, calcium chloride dihydrate, citric acid monohydrate, GenAqua (sodium perborate), magnesium chloride hexahydrate, phosphonic acid, potassium chloride, purified water, and sodium chloride
Questions?
call toll-free 1-866-393-6336, weekdays,
8:30 AM - 5:00 PM EST.
Serious side effects associated with use of this product may be reported to this number.
PRINCIPAL DISPLAY PANEL
PACKAGE LABELING
The product packaging shown below represents a sample of that currently in use. Additional packaging may also be available.
GenTeal
Moderate to Severe Dry Eye Relief
Liquid Gel Drops
Strength of a Gel,
Convenience of a Drop
Preservative Free in the EyeUSE ONLY IF TAMPER EVIDENT SEAL MARKED NOVARTIS IS INTACT.
| GENTEAL
MODERATE TO SEVERE GEL DROPS
hypromellose gel |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part349 | 09/14/2009 | |
| Labeler - Novartis Pharmaceuticals Corporation (002147023) |