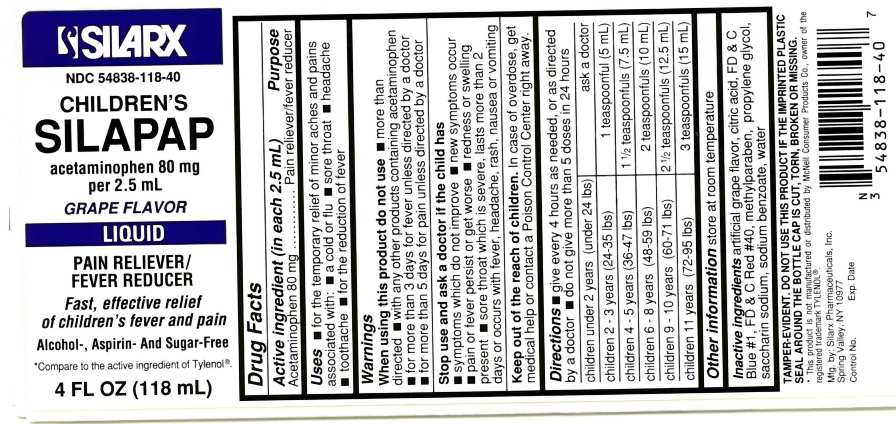
CHILDRENS SILAPAP LIQUID-GRAPE FLAVOR
-
acetaminophen liquid
Silarx Pharmaceuticals, Inc
----------
Children's Silapap Liquid-Grape FlavorDrug Facts
Active ingredient (in each 2.5 mL) Purpose
Acetaminophen 80 mg Pain reliever/fever reducer
Uses
- for the temporary relief of minor aches and pains associated with:
- a cold flu
- sore throat
- headache
- toothache
- for the reduction of fever
Warnings
When using this product do not use
- more than directed
- with any other products containing acetaminophen
- for more than 3 days for fever unless directed by a doctor
- for more than 5 days for pain unless directed by a doctor
Stop use and ask a doctor if the child has
- symptoms which do not improve
- new symptoms occur
- pain or fever persist or get worse
- redness or swelling present
- sore throat which is severe, lasts more than 2 days or occurs with fever, headache, rash, nausea or vomiting
Keep out reach of the reach of children. In case of overdose, get medical help or contact a Poison Control Center right away
Directions
- give every 4 hours as needed, or as directed by a doctor
- do not give more than 5 doses in 24 hours
| children under 2 years (under 24 lbs) | ask a doctor |
| children 2-3 years(24-35 lbs) | 1 teaspoonful (5 mL) |
| children 4-5 years (36-47 lbs) | 1 1/2 teaspoonfuls (7.5 mL) |
| children 6-8 years (48-59 lbs) | 2 teaspoonfuls (12.5 mL) |
| children 9-10 years (60-71 lbs) | 2 1/2 teaspoonfuls (12.5) |
| children 11 years (72-95 lbs) | 3 teaspoonfuls (15 mL) |
store at room temperature
Inactive ingredients
artificial grape flavor, citric acid, FD&C Blue #1, FD&C Red #40, methylparaben, propylene glycol, saccharin sodium, sodium benzoate, water
This product is not manufactured or distributed by McNeil Consumer Products., owner of the registered trademark TYLENOL®
Mfg. by:
Silarx Pharmaceuticals, Inc.
Spring Valley, NY 10977
| CHILDRENS SILAPAP LIQUID-GRAPE FLAVOR
childrens silapap liquid-grape flavor liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part341 | 09/01/1999 | 02/20/2004 |
| Labeler - Silarx Pharmaceuticals, Inc (161630033) |