temazepam (Temazepam) capsule
[Mylan Pharmaceuticals Inc.]
CIV
15 mg and 30 mg
Rx only
DESCRIPTION
Temazepam is a benzodiazepine hypnotic agent. The chemical name is 7-chloro-1,3-dihydro-3-hydroxy-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one. Its structural formula may be represented as follows:
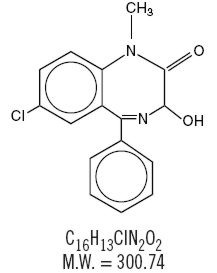
Temazepam is a white, crystalline substance, very slightly soluble in water and sparingly soluble in alcohol, USP. Temazepam capsules, 15 mg and 30 mg, are for oral administration. Each capsule contains colloidal silicon dioxide, gelatin, magnesium stearate, microcrystalline cellulose, powdered cellulose, sodium lauryl sulfate, titanium dioxide, FD&C yellow #6. Additionally, the 30 mg capsule contains D&C yellow #10.
CLINICAL PHARMACOLOGY
Pharmacokinetics
In a single and multiple dose absorption, distribution, metabolism, and excretion (ADME) study, using 3H labeled drug, temazepam was well absorbed and found to have minimal (8%) first pass metabolism. There were no active metabolites formed and the only significant metabolite present in blood was the O-conjugate. The unchanged drug was 96% bound to plasma proteins. The blood level decline of the parent drug was biphasic with the short half-life ranging from 0.4 to 0.6 hours and the terminal half-life from 3.5 to 18.4 hours (mean 8.8 hours), depending on the study population and method of determination. Metabolites were formed with a half-life of 10 hours and excreted with a half-life of approximately 2 hours. Thus, formation of the major metabolite is the rate limiting step in the biodisposition of temazepam. There is no accumulation of metabolites. A dose-proportional relationship has been established for the area under the plasma concentration/time curve over the 15 to 30 mg dose range.
Temazepam was completely metabolized through conjugation prior to excretion; 80% to 90% of the dose appeared in the urine. The major metabolite was the O-conjugate of temazepam (90%); the O-conjugate of N-desmethyl temazepam was a minor metabolite (7%).
Bioavailability, Induction, and Plasma Levels
Following ingestion of a 30 mg temazepam capsule, measurable plasma concentrations were achieved 10 to 20 minutes after dosing with peak plasma levels ranging from 666 to 982 ng/mL (mean 865 ng/mL) occurring approximately 1.2 to 1.6 hours (mean 1.5 hours) after dosing.
In a 7 day study, in which subjects were given a 30 mg capsule of a marketed bioequivalent temazepam product 1 hour before retiring, steady state (as measured by the attainment of maximal trough concentrations) was achieved by the third dose. Mean plasma levels of temazepam (for days 2 to 7) were 260 ± 210 ng/mL at 9 hours and 75 ± 80 ng/mL at 24 hours after dosing. A slight trend toward declining 24 hour plasma levels was seen after day 4 in the study, however, the 24 hour plasma levels were quite variable.
At a dose of 30 mg once-a-day for 8 weeks, no evidence of enzyme induction was found in man.
Elimination Rate of Benzodiazepine Hypnotics and Profile of Common Untoward Effects
The type and duration of hypnotic effects and the profile of unwanted effects during administration of benzodiazepine hypnotics may be influenced by the biologic half-life of the administered drug and for some hypnotics, the half-life of any active metabolites formed. Benzodiazepine hypnotics have a spectrum of half-lives from short (< 4 hours) to long (> 20 hours). When half-lives are long, drug (and for some drugs their active metabolites) may accumulate during periods of nightly administration and be associated with impairments of cognitive and/or motor performance during waking hours; the possibility of interaction with other psychoactive drugs or alcohol will be enhanced. In contrast, if half-lives are shorter, drug (and, where appropriate, its active metabolites) will be cleared before the next dose is ingested, and carry-over effects related to excessive sedation or CNS depression should be minimal or absent. However, during nightly use for an extended period, pharmacodynamic tolerance or adaptation to some effects of benzodiazepine hypnotics may develop. If the drug has a short elimination half-life, it is possible that a relative deficiency of the drug, or, if appropriate, its active metabolites (i.e., in relationship to the receptor site) may occur at some point in the interval between each night's use. This sequence of events may account for 2 clinical findings reported to occur after several weeks of nightly use of rapidly eliminated benzodiazepine hypnotics, namely, increased wakefulness during the last third of the night, and the appearance of increased signs of daytime anxiety.
Controlled Trials Supporting Efficacy
Temazepam improved sleep parameters in clinical studies. Residual medication effects ("hangover") were essentially absent. Early morning awakening, a particular problem in the geriatric patient, was significantly reduced.
Patients with chronic insomnia were evaluated in 2 week, placebo controlled sleep laboratory studies with temazepam at doses of 7.5 mg, 15 mg and 30 mg, given 30 minutes prior to bedtime. There was a linear dose-response improvement in total sleep time and sleep latency, with significant drug-placebo differences at 2 weeks occurring only for total sleep time at the 2 higher doses, and for sleep latency only at the highest dose.
In these sleep laboratory studies, REM sleep was essentially unchanged and slow wave sleep was decreased. No measurable effects on daytime alertness or performance occurred following temazepam treatment or during the withdrawal period, even though a transient sleep disturbance in some sleep parameters was observed following withdrawal of the higher doses. There was no evidence of tolerance development in the sleep laboratory parameters when patients were given temazepam nightly for at least 2 weeks.
In addition, normal subjects with transient insomnia associated with first night adaptation to the sleep laboratory were evaluated in 24 hour, placebo controlled sleep laboratory studies with temazepam at doses of 7.5 mg, 15 mg and 30 mg, given 30 minutes prior to bedtime. There was a linear dose-response improvement in total sleep time, sleep latency and number of awakenings, with significant drug-placebo differences occurring for sleep latency at all doses, for total sleep time at the 2 higher doses and for number of awakenings only at the 30 mg dose.
INDICATIONS AND USAGE
Temazepam is indicated for the short-term treatment of insomnia (generally 7 to 10 days). For patients in whom the drug is used for more than 2 to 3 weeks, periodic reevaluation is recommended to determine whether there is a continuing need. (See WARNINGS.)
For patients with short-term insomnia, instructions in the prescription should indicate that temazepam should be used for short periods of time (7 to 10 days).
Temazepam should not be prescribed in quantities exceeding a 1-month supply.
Insomnia is characterized by complaints of difficulty in falling asleep, frequent nocturnal awakenings, and/or early morning awakenings. Both sleep laboratory and outpatient studies provide support for the effectiveness of temazepam administered 30 minutes before bedtime in decreasing sleep latency and improving sleep maintenance in patients with chronic insomnia. In addition, sleep laboratory studies have confirmed similar effects in normal subjects with transient insomnia (see CLINICAL PHARMACOLOGY).
CONTRAINDICATIONS
Benzodiazepines may cause fetal damage when administered during pregnancy. An increased risk of congenital malformations associated with the use of diazepam and chlordiazepoxide during the first trimester of pregnancy has been suggested in several studies. Transplacental distribution has resulted in neonatal CNS depression following the ingestion of therapeutic doses of a benzodiazepine hypnotic during the last weeks of pregnancy.
Reproduction studies in animals with temazepam were performed in rats and rabbits. In a perinatal-postnatal study in rats, oral doses of 60 mg/kg/day resulted in increasing nursling mortality. Teratology studies in rats demonstrated increased fetal resorptions at doses of 30 and 120 mg/kg in one study and increased occurrence of rudimentary ribs, which are considered skeletal variants, in a second study at doses of 240 mg/kg or higher. In rabbits, occasional abnormalities such as exencephaly and fusion or asymmetry of ribs were reported without dose relationship. Although these abnormalities were not found in the concurrent control group, they have been reported to occur randomly in historical controls. At doses of 40 mg/kg or higher, there was an increased incidence of the 13th rib variant when compared to the incidence in concurrent and historical controls.
Temazepam is contraindicated in pregnant women. If there is a likelihood of the patient becoming pregnant while receiving temazepam, she should be warned of the potential risk to the fetus. Patients should be instructed to discontinue the drug prior to becoming pregnant. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered.
WARNINGS
Sleep disturbance may be the presenting manifestation of an underlying physical and/or psychiatric disorder. Consequently, a decision to initiate symptomatic treatment of insomnia should only be made after the patient has been carefully evaluated.
The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness.
Worsening of insomnia may be the consequence of an unrecognized psychiatric or physical disorder as may the emergence of new abnormalities of thinking or behavior. Such abnormalities have also been reported to occur in association with the use of drugs with central nervous system depressant activity, including those of the benzodiazepine class. Some of these changes may be characterized by decreased inhibition, e.g., aggressiveness and extroversion that seem out of character, similar to that seen with alcohol. Other kinds of behavioral changes can also occur, for example, bizarre behavior, agitation, hallucinations, depersonalization, and, in primarily depressed patients, the worsening of depression, including suicidal thinking. In controlled clinical trials involving 1076 patients on temazepam and 783 patients on placebo, reports of hallucinations, agitation, and overstimulation occurred at rates less than 1 in 100 patients. Hallucinations were reported in 2 temazepam patients and 1 placebo patient; agitation was reported in 1 temazepam patient; 2 temazepam patients reported overstimulation. There were no reports of worsening of depression or suicidal ideation, aggressiveness, extroversion, bizarre behavior or depersonalization in these controlled clinical trials.
It can rarely be determined with certainty whether a particular instance of the abnormal behaviors listed above is drug induced, spontaneous in origin, or a result of an underlying psychiatric or physical disorder. Nonetheless, the emergence of any new behavioral sign or symptom of concern requires careful and immediate evaluation.
Because some of the worrisome adverse effects of benzodiazepines, including temazepam, appear to be dose related (see PRECAUTIONS and DOSAGE AND ADMINISTRATION), it is important to use the lowest possible effective dose. Elderly patients are especially at risk.
Patients receiving temazepam should be cautioned about possible combined effects with alcohol and other CNS depressants.
Withdrawal symptoms (of the barbiturate type) have occurred after the abrupt discontinuation of benzodiazepines (see DRUG ABUSE AND DEPENDENCE).
PRECAUTIONS
General
Since the risk of development of oversedation, dizziness, confusion, and/or ataxia increases substantially with larger doses of benzodiazepines in elderly and debilitated patients, 7.5 mg of temazepam is recommended as the initial dosage for such patients.
Temazepam should be administered with caution in severely depressed patients or those in whom there is any evidence of latent depression; it should be recognized that suicidal tendencies may be present and protective measures may be necessary.
The usual precautions should be observed in patients with impaired renal or hepatic function and in patients with chronic pulmonary insufficiency.
If temazepam is to be combined with other drugs having known hypnotic properties or CNS depressant effects, consideration should be given to potential additive effects.
The possibility of a synergistic effect exists with the co-administration of temazepam and diphenhydramine. One case of stillbirth at term has been reported 8 hours after a pregnant patient received temazepam and diphenhydramine. A cause and effect relationship has not yet been determined. (See CONTRAINDICATIONS.)
Information for Patients
The text of a patient package insert is printed at the end of this insert. To assure safe and effective use of temazepam, the information and instructions provided in this patient package insert should be discussed with patients.
Laboratory Tests
The usual precautions should be observed in patients with impaired renal or hepatic function and in patients with chronic pulmonary insufficiency. Abnormal liver function tests as well as blood dyscrasias have been reported with benzodiazepines.
Drug Interactions
The pharmacokinetic profile of temazepam does not appear to be altered by orally administered cimetidine dosed according to labeling.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies were conducted in rats at dietary temazepam doses up to 160 mg/kg/day for 24 months and in mice at dietary dose of 160 mg/kg/day for 18 months. No evidence of carcinogenicity was observed although hyperplastic liver nodules were observed in female mice exposed to the highest dose. The clinical significance of this finding is not known.
Fertility in male and female rats was not adversely affected by temazepam.
No mutagenicity tests have been done with temazepam.
Pregnancy
Pregnancy Category X (see CONTRAINDICATIONS).
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when temazepam is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
ADVERSE REACTIONS
During controlled clinical studies in which 1076 patients received temazepam at bedtime, the drug was well tolerated. Side effects were usually mild and transient. Adverse reactions occurring in 1% or more of patients are presented in the following table:
| Temazepam % of incidence (n=1076) | Placebo % of incidence (n=783) |
|
|---|---|---|
| Drowsiness | 9.1 | 5.6 |
| Headache | 8.5 | 9.1 |
| Fatigue | 4.8 | 4.7 |
| Nervousness | 4.6 | 8.2 |
| Lethargy | 4.5 | 3.4 |
| Dizziness | 4.5 | 3.3 |
| Nausea | 3.1 | 3.8 |
| Hangover | 2.5 | 1.1 |
| Anxiety | 2.0 | 1.5 |
| Depression | 1.7 | 1.8 |
| Dry Mouth | 1.7 | 2.2 |
| Diarrhea | 1.7 | 1.1 |
| Abdominal Discomfort | 1.5 | 1.9 |
| Euphoria | 1.5 | 0.4 |
| Weakness | 1.4 | 0.9 |
| Confusion | 1.3 | 0.5 |
| Blurred Vision | 1.3 | 1.3 |
| Nightmares | 1.2 | 1.7 |
| Vertigo | 1.2 | 0.8 |
The following adverse events have been reported less frequently (0.5% to 0.9%):
Central Nervous System: anorexia, ataxia, equilibrium loss, tremor, increased dreaming
Cardiovascular: dyspnea, palpitations
Gastrointestinal: vomiting
Musculoskeletal: backache
Special Senses: hyperhidrosis, burning eyes
Amnesia, hallucinations, horizontal nystagmus and paradoxical reactions including restlessness, overstimulation and agitation were rare (less than 0.5%).
DRUG ABUSE AND DEPENDENCE
Controlled Substance
Temazepam is a controlled substance in Schedule IV.
Abuse and Dependence
Withdrawal symptoms, similar in character to those noted with barbiturates and alcohol (convulsions, tremor, abdominal, and muscle cramps, vomiting, and sweating), have occurred following abrupt discontinuance of benzodiazepines. The more severe withdrawal symptoms have usually been limited to those patients who received excessive doses over an extended period of time. Generally milder withdrawal symptoms (e.g., dysphoria and insomnia) have been reported following abrupt discontinuance of benzodiazepines taken continuously at therapeutic levels for several months. Consequently, after extended therapy at doses higher than 15 mg, abrupt discontinuation should generally be avoided and a gradual dosage tapering schedule followed. As with any hypnotic, caution must be exercised in administering temazepam to individuals known to be addiction-prone or to those whose history suggests they may increase the dosage on their own initiative. It is desirable to limit repeated prescriptions without adequate medical supervision.
OVERDOSAGE
Manifestations of acute overdosage of temazepam can be expected to reflect the CNS effects of the drug and include somnolence, confusion, and coma, with reduced or absent reflexes, respiratory depression, and hypotension. The oral LD50 of temazepam was 1963 mg/kg in mice, 1833 mg/kg in rats, and > 2400 mg/kg in rabbits.
Treatment
If the patient is conscious, vomiting should be induced mechanically or with emetics. Gastric lavage should be employed utilizing concurrently a cuffed endotracheal tube if the patient is unconscious to prevent aspiration and pulmonary complications. Maintenance of adequate pulmonary ventilation is essential. The use of pressor agents intravenously may be necessary to combat hypotension. Fluids should be administered intravenously to encourage diuresis. The value of dialysis has not been determined. If excitation occurs, barbiturates should not be used. It should be borne in mind that multiple agents may have been ingested. Flumazenil, a specific benzodiazepine receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be used in situations when an overdose with a benzodiazepine is known or suspected. Prior to the administration of flumazenil, necessary measures should be instituted to secure airway, ventilation, and intravenous access. Flumazenil is intended as an adjunct to, not as a substitute for, proper management of benzodiazepine overdose. Patients treated with flumazenil should be monitored for re-sedation, respiratory depression, and other residual benzodiazepine effects for an appropriate period after treatment. The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users and in cyclic antidepressant overdose. The complete flumazenil package insert including CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS should be consulted prior to use.
Up-to-date information about the treatment of overdose can often be obtained from a certified Regional Poison Control Center. Telephone numbers of certified Regional Poison Control Centers are listed in the Physicians' Desk Reference.
DOSAGE AND ADMINISTRATION
While the recommended usual adult dose is 15 mg before retiring, 7.5 mg may be sufficient for some patients, and others may need 30 mg. In transient insomnia, a 7.5 mg dose may be sufficient to improve sleep latency. In elderly or debilitated patients, it is recommended that therapy be initiated with 7.5 mg until individual responses are determined.
HOW SUPPLIED
Temazepam Capsules USP, are available containing either 15 mg or 30 mg of temazepam.
The 15 mg product is a peach capsule imprinted with MYLAN 4010. They are available as follows:
NDC 0378-4010-01
bottles of 100 capsules
NDC 0378-4010-05
bottles of 500 capsules
The 30 mg product is a yellow capsule imprinted with MYLAN 5050. They are available as follows:
NDC 0378-5050-01
bottles of 100 capsules
NDC 0378-5050-05
bottles of 500 capsules
STORE AT CONTROLLED ROOM TEMPERATURE 15° to 30°C (59° to 86°F). PROTECT FROM LIGHT.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505
REV FEBRUARY 2003
TZM:R14
CIV
PATIENT INFORMATION FOR TEMAZEPAM CAPSULES, USP
Introduction: Your doctor has prescribed temazepam to help you sleep. The following information is intended to guide you in the safe use of this medicine. It is not meant to take the place of your doctor's instructions. If you have any questions about temazepam capsules be sure to ask your doctor or pharmacist.
Temazepam is used to treat different types of sleep problems, such as:
- trouble falling asleep
- waking up too early in the morning
- waking up often during the night
Some people may have more than one of these problems.
Temazepam belongs to a group of medicines known as the "benzodiazepines". There are many different benzodiazepine medicines used to help people sleep better. Sleep problems are usually temporary, requiring treatment for only a short time, usually 7 to 10 days. However, if your sleep problems continue, consult your doctor. He/she will determine whether other measures are needed to overcome your sleep problems. Some people have chronic sleep problems that may require more prolonged use of sleep medicine. However, you should not use these medicines for long periods without talking with your doctor about the risks and benefits of prolonged use.
SIDE EFFECTS: Common Side Effects: All medicines have side effects. The most common side effects of benzodiazepine sleeping medicines include:
- drowsiness
- dizziness
- lightheadedness
- difficulty with coordination
You may find that these medicines make you sleepy during the day. How drowsy you feel depends upon how your body reacts to the medicine, which benzodiazepine sleeping medicine you are taking, and how large a dose your doctor has prescribed. Day-time drowsiness is best avoided by taking the lowest dose possible that will still help you to sleep at night. Your doctor will work with you to find the dose of temazepam that is best for you.
To manage these side effects while you are taking this medicine:
- Use extreme care while doing anything that requires complete alertness, such as driving a car, operating machinery, or piloting an aircraft. As with any medicines used to help people sleep better, you should be very careful when you first start taking temazepam until you know how the medicine will affect you.
- NEVER drink alcohol while you are being treated with temazepam or any benzodiazepine medicine. Alcohol can increase the side effects of temazepam or any other benzodiazepine medicine.
- Do not take any other medicines without asking your doctor first. This includes medicines you can buy without a prescription. Some medicines can cause drowsiness and are best avoided while taking temazepam.
- Always take the exact dose of temazepam prescribed by your doctor. Never change your dose without talking to your doctor first.
SPECIAL CONCERNS: There are some special problems that may occur while taking benzodiazepine sleeping medicines.
Memory Problems: Benzodiazepine sleeping medicines may cause a special type of memory loss or "amnesia". When this occurs, a person may not remember what has happened for several hours after taking the medicine. This is usually not a problem since most people fall asleep after taking the medicine.
Memory loss can be a problem, however, when sleeping medicines are taken while traveling, such as during an airplane flight and the person wakes up before the effect of the medicine is gone. This has been called "traveler's amnesia".
Memory problems were noticed in fewer than 1 in 100 patients taking temazepam in clinical trials. Memory problems can be avoided if you take temazepam only when you are able to get a full night's sleep (7 to 8 hours) before you need to be active again. Be sure to talk to your doctor if you think you are having memory problems.
Tolerance: When benzodiazepine sleeping medicines are used every night for more than a few weeks, they may lose their effectiveness to help you sleep. This is known as "tolerance".
If tolerance to the medicine develops, other effects may occur depending upon which benzodiazepine sleeping medicine you are taking. Tolerance to benzodiazepine sleeping medicines that are shorter-acting may cause you to:
- wake up during the last third of the night
- become anxious or nervous while you are awake
These effects are less common with temazepam because it is intermediate-acting.
Dependence: All the benzodiazepine sleeping medicines can cause dependence, especially when these medicines are used regularly for longer than a few weeks or at high doses. Some people develop a need to continue taking their medicines. This is known as dependence or "addiction".
When people develop dependence, they may have difficulty stopping the benzodiazepine sleeping medicine. If the medicine is suddenly stopped the body is not able to function normally and unpleasant symptoms may occur (see WITHDRAWAL). They may find they have to keep taking the medicine either at the prescribed dose or at increasing doses just to avoid withdrawal symptoms.
All people taking benzodiazepine sleeping medicines have some risk of becoming dependent on the medicine. However, people who have been dependent on alcohol or other drugs in the past may have a higher chance of becoming addicted to benzodiazepine medicines. This possibility must be considered before using these medicines for more than a few weeks.
If you have been addicted to alcohol or drugs in the past, it is important to tell your doctor before starting temazepam or any benzodiazepine sleeping medicine.
Withdrawal: Withdrawal symptoms may occur when a benzodiazepine sleeping medicine is stopped suddenly after being used daily for a long time. But these symptoms can occur even if the medicine has been used for only a week or two.
In mild cases, withdrawal symptoms may include unpleasant feelings. In more severe cases, abdominal and muscle cramps, vomiting, sweating, shakiness, and rarely, seizures may occur. These more severe withdrawal symptoms are very uncommon.
Another problem that may occur when benzodiazepine sleeping medicines are stopped is known as "rebound insomnia". This means that a person may have more trouble sleeping the first few nights after the medicine is stopped than before starting the medicine. If you should experience rebound insomnia, do not get discouraged. This problem usually goes away on its own after 1 or 2 nights.
If you have been taking temazepam or any other benzodiazepine sleeping medicine for more than 1 or 2 weeks, do not stop taking it on your own. Your doctor may give you special directions on how to gradually decrease your dose before stopping the medicine. Always follow your doctor's directions.
Changes in Behavior and Thinking: Some people using benzodiazepine sleeping medicines have experienced unusual changes in their thinking and/or behavior, including: more outgoing or aggressive behavior than normal; loss of personal identity; confusion; strange behavior; agitation; hallucinations; worsening of depression; and suicidal thoughts.
How often these effects occur depends on several factors, such as a person's general health or the use of other medicines. Clinical studies with temazepam revealed that unusual behavior changes occurred in less than 1 in 100 patients.
It is also important to realize that it is rarely clear whether these behavior changes are caused by the medicine, an illness, or occur on their own. In fact, sleep problems that do not improve may be due to illnesses that were present before the medicine was used. If you or your family notice any changes in your behavior, or if you have any unusual or disturbing thoughts, call your doctor immediately.
Pregnancy: Certain benzodiazepines have been linked to birth defects when taken by a pregnant woman in the early months of pregnancy. These medicines can also cause sedation of the unborn baby when used during the last weeks of pregnancy.
Temazepam should not be taken at any time during pregnancy. Be sure to tell your doctor if you are pregnant, if you are planning to become pregnant, or if you become pregnant while taking temazepam.
SAFE USE OF BENZODIAZEPINE SLEEPING MEDICINES
To ensure the safe and effective use of temazepam or any other benzodiazepine sleeping medicine, you should observe the following cautions:
- Temazepam is a prescription medicine and should be used ONLY as directed by your doctor. Follow your doctor's instructions about how to take, when to take, and how long to take temazepam.
- Never use temazepam or any other benzodiazepine sleeping medicine for longer than 1 or 2 weeks without first asking your doctor.
- If you notice any unusual or disturbing thoughts or behavior during treatment with temazepam or any other benzodiazepine sleeping medicine, contact your doctor.
- Tell your doctor about any medicines you may be taking, including medicines you may buy without a prescription. You should also tell your doctor if you drink alcohol. DO NOT use alcohol while taking temazepam or any other benzodiazepine sleeping medicine.
- Do not take temazepam or any other benzodiazepine sleeping medicine unless you are able to get a full night's sleep before you must be active again. For example, temazepam or any other benzodiazepine sleeping medicine should not be taken on an overnight airplane flight of less than 7 to 8 hours since "traveler's amnesia" may occur.
- Do not increase the prescribed dose of temazepam or any other benzodiazepine sleeping medicine unless instructed by your doctor.
- Use extreme care while doing anything that requires complete alertness, such as driving a car, operating machinery, or piloting an aircraft when you first start taking temazepam or any other benzodiazepine sleeping medicine until you know whether the medicine will still have some carryover effect in you the next day.
- Be aware that you may have more sleeping problems (rebound insomnia) the first night or two after stopping temazepam or any other benzodiazepine sleeping medicine.
- Be sure to tell your doctor if you are pregnant, if you are planning to become pregnant, or if you become pregnant while taking temazepam. Temazepam or any other benzodiazepine sleeping medicine should not be taken at any time during pregnancy.
- As with all prescription medicines, never share temazepam or any other benzodiazepine sleeping medicine with anyone else. Always store temazepam or any other benzodiazepine sleeping medicine in the original container out of reach of children.
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505
REV FEBRUARY 2003
PL:TZM:R10
| Temazepam (Temazepam) | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Temazepam (Temazepam) | |||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
Revised: 02/2007