clonazepam (clonazepam) tablet
[CARACO PHARMACEUTICAL LABORATORIES, LTD]
DESCRIPTION
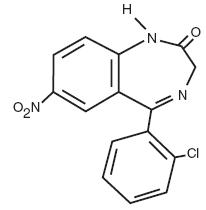
Clonazepam is a benzodiazepine and is chemically designated as 5-(2-chlorophenyl)-1,3-dihydro-7-nitro-2H-1,4-benzodiazepine-2-one. It is a light yellow crystalline powder. It has a molecular weight of 315.72 and the following structural formula.
Each tablet for oral administration, contains 0.5 mg, 1mg, or 2 mg of clonazepam. In addition each tablet contains the following inactive ingredients: Lactose Monohydrate, Microcrystalline Cellulose, Pregelatinized Starch, Magnesium Stearate, D&C Yellow No. 10 Aluminum Lake (0.5 mg only), FD&C Blue No.1 Aluminum Lake (1 mg only).
CLINICAL PHARMACOLOGY
Pharmacodynamics
The precise mechanism by which clonazepam exerts its antiseizure and antipanic effects is unknown, although it is believed to be related to its ability to enhance the activity of gamma aminobutyric acid (GABA), the major inhibitory neurotransmitter in the central nervous system. Convulsions produced in rodents by pentylenetetrazol or, to a lesser extent, electrical stimulation are antagonized, as are convulsions produced by photic stimulation in susceptible baboons. A taming effect in aggressive primates, muscle weakness and hypnosis are also produced. In humans, clonazepam is capable of suppressing the spike and wave discharge in absence seizures (petit mal) and decreasing the frequency, amplitude, duration and spread of discharge in minor motor seizures.
Pharmacokinetics
Clonazepam is rapidly and completely absorbed after oral administration. The absolute bioavailability of clonazepam is about 90%. Maximum plasma concentrations of clonazepam are reached within 1 to 4 hours after oral administration. Clonazepam is approximately 85% bound to plasma proteins. Clonazepam is highly metabolized, with less than 2% unchanged clonazepam being excreted in the urine. Biotransformation occurs mainly by reduction of the 7-nitro group to the 4-amino derivative. This derivative can be acetylated, hydroxylated and glucuronidated. Cytochrome P-450 including CYP3A, may play an important role in clonazepam reduction and oxidation. The elimination half-life of clonazepam is typically 30 to 40 hours. Clonazepam pharmacokinetics are dose-independent throughout the dosing range. There is no evidence that clonazepam induces its own metabolism or that of other drugs in humans.
Pharmacokinetics in Demographic Subpopulations and in Disease States
Controlled studies examining the influence of gender and age on clonazepam pharmacokinetics have not been conducted, nor have the effects of renal or liver disease on clonazepam pharmacokinetics been studied. Because clonazepam undergoes hepatic metabolism, it is possible that liver disease will impair clonazepam elimination. Thus, caution should be exercised when administering clonazepam to these patients.
Clinical Trials
Panic Disorder
The effectiveness of clonazepam tablets in the treatment of panic disorder was demonstrated in two double-blind, placebo-controlled studies of adult outpatients who had a primary diagnosis of panic disorder (DSM-IIIR) with or without agoraphobia. In these studies, clonazepam tablets was shown to be significantly more effective than placebo in treating panic disorder on change from baseline in panic attack frequency, the Clinician's Global Impression Severity Of Illness Score and the Clinician's Global Impression Improvement Score.
Study 1 was a 9-week, fixed dose study involving clonazepam tablet doses of 0.5, 1, 2, 3 or 4 mg/day or placebo. This study was conducted in four phases: a 1-week placebo lead-in, a 3-week upward titration, a 6-week fixed dose and a 7-week discontinuance phase. A significant difference from placebo was observed consistently only for the 1 mg/day group. The difference between the 1 mg. dose group and placebo in reduction from baseline in the number of full panic attacks was approximately 1 panic attack per week. At endpoint, 74% of patients receiving clonazepam 1 mg/day were free of full panic attacks, compared to 56% of placebo-treated patients.
Study 2 was a 6-week, flexible-dose study involving clonazepam tablets in a dose range of 0.5 to 4 mg/day or placebo. This study was conducted in three phases: a 1-week placebo lead-in, a 6-week optimal-dose and a 6-week discontinuance phase. The mean clonazepam dose during the optimal dosing period was 2.3 mg/day. The difference between clonazepam tablets and placebo in reduction from baseline in the number of full panic attacks was approximately 1 panic attack per week. At endpoint, 62% of patients receiving clonazepam were free of full panic attacks, compare to 37% of placebo-treated patients.
Subgroup analyses did not indicate that there were any differences in treatment outcomes as a function of race or gender.
INDICATIONS AND USAGE
Seizure Disorders
Clonazepam tablets are useful alone or as an adjunct in the treatment of the Lennox-Gastaut syndrome (petit mal variant), akinetic and myoclonic seizures. In patients with absence seizures (petit mal) who have failed to respond to succinimides, clonazepam tablets may be useful.
In some studies, up to 30% of patients have shown a loss of anticonvulsant activity, often within 3 months of administration. In some cases, dosage adjustment may reestablish efficacy.
Panic Disorder
Clonazepam tablets are indicated for the treatment of panic disorder, with or without agoraphobia, as defined in DSM-IV. Panic disorder is characterized by the occurrence of unexpected panic attacks and associated concern about having additional attacks, worry about the implications or consequences of the attacks, and/or a significant change in behavior related to the attacks.
The efficacy of clonazepam tablets were established in two 6- to 9-week trials in panic disorder patients whose diagnoses corresponded to the DSM-IIIR category of panic disorder (see CLINICAL PHARMACOLOGY: Clinical Trials).
Panic disorder (DSM-IV) is characterized by recurrent unexpected panic attacks, ie, a discrete period of intense fear or discomfort in which four (or more) of the following symptoms develop abruptly and reach a peak within 10 minutes:
- palpitations, pounding heart or accelerated heart rate;
- sweating;
- trembling or shaking;
- sensations of shortness of breath or smothering;
- feeling of choking;
- chest pain or discomfort;
- nausea or abdominal distress;
- feeling dizzy, unsteady, lightheaded or faint;
- derealization (feelings or unreality) or depersonalization (being detached from oneself);
- fear of losing control;
- fear of dying;
- paresthesias (numbness or tingling sensations);
- chills or hot flushes.
The effectiveness of clonazepam tablets in long-term use, that is, for more than 9 weeks, has not been systematically studied in controlled clinical trials. The physician who elects to use clonazepam tablets for extended periods should periodically reevaluate the long-term usefulness of the drug for the individual patient (see DOSAGE AND ADMINISTRATION).
CONTRAINDICATIONS
Clonazepam tablets should not be used in patients with a history of sensitivity to benzodiazepines, nor in patients with clinical or biochemical evidence of significant liver disease. It may be used in patients with open angle glaucoma who are receiving appropriate therapy but is contraindicated in acute narrow angle glaucoma.
WARNINGS
Interference with Cognitive and Motor Performance
Since clonazepam produces CNS depression, patients receiving this drug should be cautioned against engaging in hazardous occupations requiring mental alertness, such as operating machinery or driving a motor vehicle. They should also be warned about the concomitant use of alcohol or other CNS-depressant drugs during clonazepam therapy (see PRECAUTIONS: Drug Interactions and Information for Patients).
Pregnancy Risks
Data from several sources raise concerns about the use of clonazepam during pregnancy.
Animal Findings
In three studies in which clonazepam tablets were administered orally to pregnant rabbits at doses of 0.2, 1, 5 or 10 mg/kg/day (low dose approximately .2 times the maximum recommended human dose of 20 mg/day for seizure disorders and equivalent to the maximum dose of 4 mg/day for panic disorder on a mg/m2 basis) during the period of organogenesis, a similar pattern of malformations (cleft palate, open eyelid, fused sternebrae and limb defects) was observed in a low, non-dose-related incidence in exposed litters from all dosage groups. Reductions in maternal weight gain occurred at dosages of 5 mg/kg/day or greater and reduction in embryo-fetal growth occurred in one study at a dosage of 10 mg/kg/day. No adverse maternal or embryo-fetal effects were observed in mice and rats following administration during organogenesis of oral doses up to 15 mg/kg/day or 40 mg/kg/day, respectively (4 and 20 times the maximum recommended human dose of 20 mg/day for seizure disorders and 20 and 100 times the maximum dose of 4 mg/day for panic disorder, respectively on a mg/m2 basis).
General Concerns and Considerations About Anticonvulsants
Recent reports suggest an association between the use of anticonvulsant drugs by women with epilepsy and an elevated incidence of birth defects in children born to these women. Data are more extensive with respect to diphenylhydantoin and phenobarbital, but these are also the most commonly prescribed anticonvulsants; less systematic or anecdotal reports suggest a possible similar association with the use of all known anticonvulsant drugs.
In children of women treated with drugs for epilepsy, reports suggesting an elevated incidence of birth defects cannot be regarded as adequate to prove a definite cause and effect relationship. There are intrinsic methodologic problems in obtaining adequate data on drug teratogenicity in humans; the possibility also exists that other factors (eg, genetic factors or the epileptic condition itself) may be more important than drug therapy in leading to birth defects. The great majority of mothers on anticonvulsant medication deliver normal infants. It is important to note that anticonvulsant drugs should not be discontinued in patients in whom the drug is administered to prevent seizures because of the strong possibility of precipitating status epilepticus with attendant hypoxia and threat to life. In individual cases where the severity and frequency of the seizure disorder are such that the removal of medication does not pose a serious threat to the patient, discontinuation of the drug may be considered prior to and during pregnancy; however, it cannot be said with any confidence that even mild seizures do not pose some hazards to the developing embryo or fetus.
General concerns About Benzodiazepines
An increased risk of congenital malformations associated with the use of benzodiazepine drugs has been suggested in several studies.
There may also be non-teratogenic risks associated with the use of benzodiazepines during pregnancy. There have been reports of neonatal flaccidity, respiratory and feeding difficulties, and hypothermia in children born to mothers who have been receiving benzodiazepines late in pregnancy. In addition, children born to mothers receiving benzodiazepines late in pregnancy may be at some risk of experiencing withdrawal symptoms during the postnatal period.
Advice Regarding the Use of Clonazepam in Women of Childbearing Potential
In general, the use of clonazepam tablets in women of childbearing potential, and more specifically during known pregnancy, should be considered only when the clinical situation warrants the risk to the fetus.
The specific considerations addressed above regarding the use of anticonvulsants for epilepsy in women of childbearing potential should be weighed in treating or counseling these women.
Because of experience with other members of the benzodiazepine class, clonazepam tablets are assumed to be capable of causing an increased risk of congenital abnormalities when administered to a pregnant woman during the first trimester. Because use of these drugs is rarely a matter of urgency in the treatment of panic disorder, their use during the first trimester should almost always be avoided. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Patients should also be advised that if they become pregnant during therapy or intend to become pregnant, they should communicate with their physician about the desirability of discontinuing the drug.
Withdrawal Symptoms
Withdrawal symptoms of the barbiturate type have occurred after the discontinuation of benzodiazepines (see DRUG ABUSE AND DEPENDENCE section).
PRECAUTIONS
General
Worsening of Seizures: When used in patients in whom several different types of seizure disorders coexist, clonazepam tablets may increase the incidence or precipitate the onset of generalized tonic-clonic seizures (grand mal). This may require the addition of appropriate anticonvulsants or an increase in their dosages. The concomitant use of valproic acid and clonazepam tablets may produce absence status.
Laboratory Testing During Long-Term Therapy: Periodic blood counts and liver function tests are advisable during long-term therapy with clonazepam tablets.
Risks of Abrupt Withdrawal: The abrupt withdrawal of clonazepam tablets, particularly in those patients on long-term, high-dose therapy, may precipitate status epilepticus. Therefore, when discontinuing clonazepam tablets, gradual withdrawal is essential. While clonazepam tablets are being gradually withdrawn, the simultaneous substitution of another anticonvulsant may be indicated.
Caution in Renally Impaired Patients: Metabolites of clonazepam tablets are excreted by the kidneys: to avoid their excess accumulation, caution should be exercised in the administration of the drug to patients with impaired renal function.
Hypersalivation: Clonazepam tablets may produce an increase in salivation. This should be considered before giving the drug to patients who have difficulty handling secretions. Because of this and the possibility of respiratory depression, clonazepam tablets should be used with caution in patients with chronic respiratory diseases.
Information for Patients
Physicians are advised to discuss the following issues with patients for whom they prescribe clonazepam tablets.
Dose Changes: To assure the safe and effective use of benzodiazepines, patients should be informed that, since benzodiazepines may produce psychological and physical dependence, it is advisable that they consult with their physician before either increasing the dose or abruptly discontinuing this drug.
Interference with Cognitive and Motor Performance: Because benzodiazepines have the potential to impair judgement, thinking or motor skills, patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that clonazepam therapy does not affect them adversely.
Pregnancy: Patients should be advised to notify their physician if they become pregnant or intend to become pregnant during therapy with clonazepam tablets (see WARNINGS).
Nursing: Patients should be advised not to breastfeed an infant if they are taking clonazepam tablets.
Concomitant Medication: Patients should be advised to inform their physicians if they are taking, or plan to take, any prescription or over-the-counter drugs, since there is a potential for interactions.
Alcohol: Patients should be advised to avoid alcohol while taking clonazepam tablets.
Drug Interactions
Effect of clonazepam on the Pharmacokinetics of Other Drugs: Clonazepam does not appear to alter the pharmacokinetics of phenytoin, carbamazepine or phenobarbital. The effect of clonazepam on the metabolism of other drugs has not been investigated.
Effect of Other Drugs on the Pharmacokinetics of Clonazepam: Literature reports suggest that ranitidine, an agent that decreases stomach acidity, does not greatly alter clonazepam pharmacokinetics.
In a study in which 2 mg Klonapin®Wafer (clonazepam orally disintegrating tablet)*was administered with and without propantheline (an anticholinergic agent with multiple effects on the GI tract) to healthy volunteers, the AUC of clonazepam was 10% lower and the Cmax of clonazepam was 20% lower when the orally disintegrating tablet was given with propantheline compared to when it is given alone.
Fluoxetine does not affect the pharmacokinetics of clonazepam. Cytochrome P-450 inducers, such as phenytoin, carbamazepine and phenobarbital, induce clonazepam metabolism, causing an approximately 30% decrease in plasma clonazepam levels. Although clinical studies have not been performed, based on the involvement of the cytochrome P-450 3A family in clonazepam metabolism, inhibitors of this enzyme system, notably oral antifungal agents, should be used cautiously in patients receiving clonazepam.
Pharmacodynamic Interactions: The CNS-depressant action of the benzodiazepine class of drugs may be potentiated by alcohol, narcotics, barbiturates, nonbarbiturate hypnotics, antianxiety agents, the phenothiazines, thioxanthene and butyrophenone classes of antipsychotic agents, monoamine oxidase inhibitors and the tricyclic antidepressants, and by other anticonvulsant drugs.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with clonazepam.
The data currently available are not sufficient to determine the genotoxic potential of clonazepam.
In a two-generation fertility study in which clonazepam was given orally to rats at 10 and 100 mg/kg/day (low dose approximately 5 times and 24 times the maximum recommended human dose of 20 mg/day for seizure disorder and 4 mg/day for panic disorder, respectively, on a mg/m2 basis), there was a decrease in the number of pregnancies and in the number of offspring surviving until weaning.
Pregnancy: Teratogenic Effects
Pregnancy Category D (see WARNINGS).
Labor and Delivery
The effect of clonazepam tablets on labor and delivery in humans has not been specifically studied; however, perinatal complications have been reported in children born to mothers who have been receiving benzodiazepines late in pregnancy, including findings suggestive of either excess benzodiazepine exposure or of withdrawal phenomena (see WARNINGS: Pregnancy Risks).
Nursing Mothers
Mothers receiving clonazepam tablets should not breastfeed their infants.
Pediatric Use
Because of the possibility that adverse effects on physical or mental development could become apparent only after many years, a benefit-risk consideration of the long-term use of clonazepam tablets is important in pediatric patients being treated for seizure disorder (see INDICATIONS AND USAGE and DOSAGE AND ADMINISTRATION).
Safety and effectiveness in pediatric patients with panic disorder below the age of 18 have not been established.
Geriatric Use
Clinical studies of clonazepam tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Because clonazepam tablets undergoes hepatic metabolism, it is possible that liver disease will impair clonazepam elimination. Metabolites of clonazepam are excreted by the kidneys; to avoid their excess accumulation, caution should be exercised in the administration of the drug to patients with impaired renal function. Because elderly patients are more likely to have decreased hepatic and/or renal function, care should be taken in dose selection, and it may be useful to assess hepatic and/or renal function at the time of dose selection.
Sedating drugs may cause confusion and over-sedation in the elderly; elderly patients generally should be started on low doses of clonazepam tablets and observed closely.
ADVERSE REACTIONS
The adverse reactions for clonazepam tablets are provided separately for patients with seizure disorders and with panic disorders.
Seizure disorders
The most frequently occurring side effects of clonazepam tablets are referable to CNS depression. Experience in treatment of seizures has shown that drowsiness has occurred in approximately 50% of patients and ataxia in approximately 30%. In some cases, these may diminish with time; behavior problems have been noted in approximately 25% of patients. Others, listed by system, are:
Neurologic: Abnormal eye movements, aphonia, choreiform movements, coma, diplopia, dysarthria, dysdiadochokinesis, “glassy-eyed” appearance, headache, hemiparesis, hypotonia, nystagmus, respiratory depression, slurred speech, tremor, vertigo.
Psychiatric: Confusion, depression, amnesia, hallucinations, hysteria, increased libido, insomnia, psychosis, suicidal attempt (the behavior effects are more likely to occur in patients with a history of psychiatric disturbances). The following paradoxical reactions have been observed: excitability, irritability, aggressive behavior, agitation, nervousness, hostility, anxiety, sleep disturbances, nightmares and vivid dreams.
Respiratory: Chest congestion, rhinorrhea, shortness of breath, hypersecretion in upper respiratory passages.
Cardiovascular: Palpitations.
Dermatologic: Hair loss, hirsutism, skin rash, ankle and facial edema.
Gastrointestinal: Anorexia, coated tongue, constipation, diarrhea, dry mouth, encopresis, gastritis, increased appetite, nausea, sore gums.
Genitourinary: Dysuria, enuresis, nocturia, urinary retention.
Musculoskeletal: Muscle weakness, pains.
Miscellaneous: Dehydration, general deterioration, fever, lymphadenopathy, weight loss or gain.
Hematopoietic: Anemia, leukopenia, thrombocytopenia, eosinophilia.
Hepatic: Hepatomegaly, transient elevations of serum transaminases and alkaline phosphatase.
Panic disorder
Adverse events during exposure to clonazepam tablets were obtained by spontaneous report and recorded by clinical investigators using terminology of their own choosing. Consequently, it is not possible to provide a meaningful estimate of the proportion of individuals experiencing adverse events without first grouping similar types of events into a smaller number of standardized event categories. In the tables and tabulations that follow, CIGY dictionary terminology has been used to classify reported adverse events, except in certain cases in which redundant terms were collapsed into more meaningful terms, as noted below.
The stated frequencies of adverse events represent the proportion of individuals who experienced, at least once, a treatment-emergent adverse event of the type listed. An event was considered treatment-emergent if it occurred for the first time or worsened while receiving therapy following baseline evaluation.
Adverse Findings Observed In Short-Term, Placebo-Controlled Trials: Adverse Events Associated with Discontinuation of Treatment
Overall, the incidence of discontinuation due to adverse events was 17% in clonazepam tablets compared to 9% for placebo in the combined data of two 6- to 9-week trials. The most common events (≥1%) associated with discontinuation and a dropout rate twice or greater for clonazepam tablets than that of placebo included the following:
| Adverse Event | Clonazepam Tablets (N-574) | Placebo (N=294) |
|---|---|---|
| Somnolence | 7% | 1% |
| Depression | 4% | 1% |
| Dizziness | 1% | <1% |
| Nervousness | 1% | 0% |
| Ataxia | 1% | 0% |
| Intellectual Ability Reduced | 1% | 0% |
Adverse Events Occurring at an Incidence of 1% or More Among Clonazepam-Treated Patients:
Table 1 enumerates the incidence, rounded to the nearest percent, of treatment-emergent adverse events that occurred during acute therapy of panic disorder from a pool of two 6- to 9-week trials. Events reported in 1% or more of patients treated with clonazepam tablets (doses ranging from 0.5 to 4 mg/day) and for which the incidence was greater than that in placebo-treated patients are included.
The prescriber should be aware that the figures in Table 1 cannot be used to predict the incidence of side effects in the course of usual medical practice where patient characteristics and other factors differ from those that prevailed in the clinical trials.
Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different treatments, uses and investigators. The cited figures, however, do provide the prescribing physician with some basis for estimating the relative contribution of drug and nondrug factors to the side effect incidence in the population studied.
| Adverse Event by Body System | Clonazepam Maximum Daily Dose | All Clonazepam tablets Groups N=574 % | Placebo N=294 % |
||||
|---|---|---|---|---|---|---|---|
| <1 mg n=96 % | 1-<2mg n=129 % | 2-<3mg n=113 % | ≥3mg n=235 % |
||||
|
* Events reported by at least 1% of patients treated with clonazepam tablets and for which the incidence was greater than that for placebo. † Indicates that the p-value for the dose-trend test (Cochran-Mantel-Haenszel) for adverse event incidence was ≤ 0.10. ‡ Denominators for events in gender-specific systems are n=240 (clonazepam), 102 (placebo) for male, and 334 (clonazepam), 192 (placebo) for female. |
|||||||
| Central & Peripheral Nervous System | |||||||
| Somnolence† | 26 | 35 | 50 | 36 | 37 | 10 | |
| Dizziness | 5 | 5 | 12 | 8 | 8 | 4 | |
| Coordination Abnormal† | 1 | 2 | 7 | 9 | 6 | 0 | |
| Ataxia† | 2 | 1 | 8 | 8 | 5 | 0 | |
| Dysarthria† | 0 | 0 | 4 | 3 | 2 | 0 | |
| Psychiatric | |||||||
| Depression | 7 | 6 | 8 | 8 | 7 | 1 | |
| Memory Disturbance | 2 | 5 | 2 | 5 | 4 | 2 | |
| Nervousness | 1 | 4 | 3 | 4 | 3 | 2 | |
| Intellectual Ability Reduced | 0 | 2 | 4 | 3 | 2 | 0 | |
| Emotional Lability | 0 | 1 | 2 | 2 | 1 | 1 | |
| Libido Decreased | 0 | 1 | 3 | 1 | 1 | 0 | |
| Confusion | 0 | 2 | 2 | 1 | 1 | 0 | |
| Respiratory System | |||||||
| Upper Respiratory Tract Infection† | 10 | 10 | 7 | 6 | 8 | 4 | |
| Sinusitis | 4 | 2 | 8 | 4 | 4 | 3 | |
| Rhinitis | 3 | 2 | 4 | 2 | 2 | 1 | |
| Coughing | 2 | 2 | 4 | 0 | 2 | 0 | |
| Pharyngitis | 1 | 1 | 3 | 2 | 2 | 1 | |
| Bronchitis | 1 | 0 | 2 | 2 | 1 | 1 | |
| Gastrointestinal System | |||||||
| Constipation† | 0 | 1 | 5 | 3 | 2 | 2 | |
| Appetite Decreased | 1 | 1 | 0 | 3 | 1 | 1 | |
| Abdominal Pain† | 2 | 2 | 2 | 0 | 1 | 1 | |
| Body As a Whole | |||||||
| Fatigue | 9 | 6 | 7 | 7 | 7 | 4 | |
| Allergic Reaction | 3 | 1 | 4 | 2 | 2 | 1 | |
| Musculoskeletal | |||||||
| Myalgia | 2 | 1 | 4 | 0 | 1 | 1 | |
| Resistance Mechanism Disorders | |||||||
| Influenza | 3 | 2 | 5 | 5 | 4 | 3 | |
| Urinary System | |||||||
| Micturition Frequency | 1 | 2 | 2 | 1 | 1 | 0 | |
| Urinary Tract Infection† | 0 | 0 | 2 | 2 | 1 | 0 | |
| Vision Disorders | |||||||
| Blurred Vision | 1 | 2 | 3 | 0 | 1 | 1 | |
| Reproduction Disorders‡ | |||||||
| Female | |||||||
| Dysmenorrhea | 0 | 6 | 5 | 2 | 3 | 2 | |
| Colpitis | 4 | 0 | 2 | 1 | 1 | 1 | |
| Male | |||||||
| Ejaculation Delayed | 0 | 0 | 2 | 2 | 1 | 0 | |
| Impotence | 3 | 0 | 2 | 1 | 1 | 0 | |
Commonly Observed Adverse Events:
| Adverse Event | Clonazepam (N=574) | Placebo (N=294) |
|---|---|---|
|
* Treatment -emergent events for which the incidence in the clonazepam patients was ≥ 5% and at least twice that in the placebo patients. |
||
| Somnolence | 37% | 10% |
| Depression | 7% | 1% |
| Coordination Abnormal | 6% | 0% |
| Ataxia | 5% | 0% |
Treatment-emergent Depressive Symptoms: In the pool of two short-term placebo-controlled trials, adverse events classified under the preferred term “depression” were reported in 7% of clonazepam tablets treated patients compared to 1% of placebo-treated patients, without any clear pattern of dose relatedness. In these same trials, adverse events classified under the preferred term “depression” were reported as leading to discontinuation in 4% of clonazepam tablets treated patients compared to 1% of placebo-treated patients. While these findings are noteworthy, Hamilton Depression Rating Scale (HAM-D) data collected in these trials revealed a larger decline in HAM-D scores in the clonazepam group than the placebo group suggesting that clonazepam-treated patients were not experiencing a worsening or emergency of clinical depression.
Other Adverse Events Observed During the Premarketing Evaluation of clonazepam tablets in Panic Disorder:
Following is a list of modified CIGY terms that reflect treatment-emergent adverse events reported by patients treated with clonazepam tablets at multiple doses during clinical trials. All reported events are included except those already listed in Table 1 or elsewhere in labeling, those events for which a drug cause was remote, those event terms which were so general as to be uninformative, and events reported only once and which did not have a substantial probability of being acutely life-threatening. It is important to emphasize that, although the events occurred during treatment with clonazepam tablets, they were not necessarily caused by it.
Events are further categorized by body system and listed in order of decreasing frequency. These adverse events were reported infrequently, which is defined as occurring in 1/100 to 1/1000 patients.
Body as a whole: weight increase, accident, weight decrease, wound, edema, fever, shivering, abrasions, ankle edema, edema foot, edema periorbital, injury, malaise, pain, cellulitis, inflammation localized.
Cardiovascular Disorders: chest pain, hypotension postural.
Central & Peripheral Nervous System Disorders: migraine, paresthesia, drunkenness feeling of enuresis, paresis, tremor, burning skin, falling, head fullness, hoarseness, hyperactivity, hypoesthesia, tongue thick, twitching.
Gastrointestinal System Disorders: abdominal discomfort, gastrointestinal inflammation, stomach upset, toothache, flatulence, pyrosis, saliva increased, tooth disorder, bowel movements frequent, pain pelvic, dyspepsia, hemorrhoids.
Hearing and Vestibular Disorders: vertigo, otitis, earache, motion sickness.
Heart Rate and Rhythm Disorders: palpitation.
Metabolic and Nutritional Disorders: thirst, gout.
Musculoskeletal System Disorders: back pain, fracture traumatic, sprains and strains, pain leg, pain nape, cramps muscle, cramps leg, pain ankle, pain shoulder, tendinitis, arthralgia, hypertonia, lumbago, pain feet, pain jaw, pain knee, swelling knee.
Platelet, Bleeding & Clotting Disorders: bleeding dermal.
Psychiatric Disorders: Insomnia, organic disinhibition, anxiety, depersonalization, dreaming excessive, libido loss, appetite increased, libido increased, reactions decreased, aggressive reaction, apathy, attention lack, excitement, feeling mad, hunger abnormal, illusion, nightmares, sleep disorder, suicide ideation, yawning.
Reproductive Disorders, Female: breast pain, menstrual irregularity.
Reproductive Disorders, Male: ejaculation decreased.
Resistance Mechanism Disorders: infection mycotic, infection viral, infection streptococcal, herpes simplex infection, infectious mononucleosis, moniliasis.
Respiratory System Disorders: sneezing excessive, asthmatic attack, dyspnea, nosebleed, pneumonia, pleurisy.
Skin and Appendages Disorder: acne flare, alopecia, xeroderma, dermatitis contact, flushing, pruritis, pustular reaction, skin burns, skin disorder.
Special Senses Other Disorders: taste loss
Urinary System Disorders: dysuria, cystitis, polyuria, urinary incontinence, bladder dysfunction, urinary retention, urinary tract bleeding, urine discoloration.
Vascular (Extracardiac) Disorders: thrombophlebitis leg.
Vision Disorders: eye irritation, visual disturbance, diplopia, eye twitching, styes, visual field defect, xerophthalmia.
DRUG ABUSE AND DEPENDENCE
Controlled Substance Class
Clonazepam is a Schedule IV controlled substance.
Physical and Psychological Dependence
Withdrawal symptoms, similar in character to those noted with barbiturates and alcohol (eg, convulsions, psychosis, hallucinations, behavioral disorder, tremor, abdominal and muscle cramps) have occurred following abrupt discontinuance of clonazepam. The more severe withdrawal symptoms have usually been limited to those patients who received excessive doses over an extended period of time. Generally milder withdrawal symptoms (eg, dysphoria and insomnia) have been reported following abrupt discontinuance of benzodiazepines taken continuously at therapeutic levels for several months. Consequently, after extended therapy, abrupt discontinuation should generally be avoided and a gradual dosage tapering schedule followed (see DOSAGE AND ADMINISTRATION section). Addiction-prone individuals (such as drug addicts or alcoholics) should be under careful surveillance when receiving clonazepam or other psychotropic agents because of the predisposition of such patients to habituation and dependence.
Following the short-term treatment of patients with panic disorder in Studies 1 and 2 (see CLINICAL PHARMACOLOGY: Clinical Trials), patients were gradually withdrawn during a 7-week downward-titration (discontinuance) period. Overall, the discontinuance period was associated with good tolerability and a very modest clinical deterioration, without evidence of a significant rebound phenomenon. However, there are not sufficient data from adequate and well-controlled long-term clonazepam studies in patients with panic disorder to accurately estimate the risks of withdrawal symptoms and dependence that may be associated with such use.
OVERDOSAGE
Human Experience
Symptoms of clonazepam overdosage, like those produced by other CNS depressants, include somnolence, confusion, coma, and diminished reflexes.
Overdose management
Treatment includes monitoring of respiration, pulse and blood pressure, general supportive measures and immediate gastric lavage. Intravenous fluids should be administered and an adequate airway maintained. Hypotension may be combated by the use of levarterenol or metaraminol. Dialysis is of no known value.
Flumazenil, a specific benzodiazepine-receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be used in situations when an overdose with a benzodiazepine is known or suspected. Prior to the administration of flumazenil, necessary measures should be instituted to secure airway, ventilation and intravenous access. Flumazenil is intended as an adjunct to, not as a substitute for, proper management of benzodiazepine overdose. Patients treated with flumazenil should be monitored for resedation, respiratory depression and other residual benzodiazepine effects for an appropriate period after treatment. The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users and in cyclic antidepressant overdose. The complete flumazenil package insert, including CONTRAINDICATIONS, WARNINGS and PRECAUTIONS, should be consulted prior to use.
Flumazenil is not indicated in patients with epilepsy who have been treated with benzodiazepines. Antagonism of the benzodiazepine effect in such patients may provoke seizures.
Serious sequelae are rare unless other drugs or alcohol have been taken concomitantly.
DOSAGE AND ADMINISTRATION
Clonazepam is available as a tablet. The tablets should be administered with water by swallowing the tablet whole.
Seizure disorders
Adults: The initial dose for adults with seizure disorders should not exceed 1.5 mg/day divided into three doses. Dosage may be increased in increments of 0.5 to 1 mg every 3 days until seizures are adequately controlled or until side effects preclude any further increase. Maintenance dosage must be individualized for each patient depending upon response. Maximum recommended daily dose is 20 mg.
The use of multiple anticonvulsants may result in an increase of depressant adverse effects. This should be considered before adding clonazepam tablets to an existing anticonvulsant regimen.
Pediatric Patients: Clonazepam tablets are administered orally. In order to minimize drowsiness, the initial dose for infants and children (up to 10 years of age or 30 kg of body weight) should be between 0.01 and 0.03 mg/kg/day but not to exceed 0.05 mg/kg/day given in two or three divided doses. Dosage should be increased by no more than 0.25 to 0.5 mg every third day until a daily maintenance dose of 0.1 to 0.2 mg/kg of body weight has been reached, unless seizures are controlled or side effects preclude further increase. Whenever possible, the daily dose should be divided into three equal doses. If doses are not equally divided, the largest dose should be given before retiring.
Geriatric Patients: There is no clinical trial experience with clonazepam tablets in panic disorder patients 65 years of age and older. In general, elderly patients should be started on low doses of clonazepam tablets and observed closely (see Precautions: Geriatric Use).
Panic Disorder
Adults: The initial dose for adults with panic disorder is 0.25 mg bid. An increase to the target dose for most patients of 1 mg/day may be made after 3 days. The recommended dose of 1 mg/day is based on the results from a fixed dose study in which the optimal effect was seen at 1 mg/day. Higher doses of 2, 3 and 4 mg/day in that study were less effective than the 1 mg/day dose and were associated with more adverse effects. Nevertheless, it is possible that some individual patients may benefit from doses of up to a maximum dose of 4 mg/day, and in those instances, the dose may be increased in increments of 0.125 to 0.25 mg bid every 3 days until panic disorder is controlled or until side effects make further increases undesired. To reduce the inconvenience of somnolence, administration of one dose at bedtime may be desirable.
Treatment should be discontinued gradually, with a decrease of 0.125 mg bid every 3 days, until the drug is completely withdrawn.
There is no body of evidence available to answer the question of how long the patient treated with clonazepam should remain on it. Therefore, the physician who elects to use clonazepam tablets for extended periods should periodically reevaluate the long-term usefulness of the drug for the individual patient.
Pediatric Patients: There is no clinical trial experience with clonazepam tablets in panic disorder patients under 18 years of age.
Geriatric Patients: There is no clinical trial experience with clonazepam tablets in seizure disorder patients 65 years of age and older. In general, elderly patients should be started on low doses of clonazepam tablets and observed closely (see PRECAUTIONS: Geriatric Use).
HOW SUPPLIED
Clonazepam Tablets USP, 0.5 mg are round, yellow, scored and debossed “.5” on one side and “273” on the other side, in bottles of 100 (NDC 57664-273-08), Bottles of 500 (57664-273-13) and Bottles of 1000 (NDC 57664-273-18).
Clonazepam Tablets USP, 1 mg are round, blue, scored and debossed “1” on one side and “274” on the other side, in bottles of 100 (NDC 57664-274-08), Bottles of 500 (NDC 57664-274-13) and Bottles of 1000 (NDC 57664-274-18).
Clonazepam Tablets USP, 2 mg are round, white, scored and debossed “2” on one side and “275” on the other side, in bottles of 100 (NDC 57664 275-08), Bottles of 500 (NDC-57664-275-13) and Bottles of 1000 (NDC 57664-275-18).
Store at controlled room temperature 15-30°C (59-86°F).
Dispense in tight, light resistant containers as defined in USP.
Klonapin® is a registered trademark of Roche Pharmaceuticals.
C.S. No. : 5151T06
Issued 07/06
CARACO PHARMACEUTICAL LABORATORIES, LTD.
DETROIT, MI 48202
| Clonazepam (clonazepam) | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Clonazepam (clonazepam) | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Clonazepam (clonazepam) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Revised: 09/2006