DIPHENHYDRAMINE HYDROCHLORIDE
-
diphenhydramine hydrochloride capsule
BANOPHEN
-
diphenhydramine hydrochloride capsule
Major Pharmaceuticals
----------
Diphenhydramine Hydrochloride CapsulesActive ingredient
Diphenhydramine Hydrochloride 25 mg
Purpose
Antihistamine
Use
Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies and common cold, sneezing, runny nose, itchy, watery eyes, itchy throat and nose.
Warning
Ask a doctor before use if you have glaucoma, a breathing problem such as emphysema or chronic bronchitis, trouble urinating due to an enlarged prostate gland.
Ask a doctor before use if you have
glaucoma, a breathing problem such as emphysema or chronic bronchitis, trouble urinating due to an enlarged prostate gland.
Ask a doctor or pharmacist before use if you are
taking taking tranquilizers or sedative.
When using this product
you may get drowsy; avoid alcoholic drinks. Alcohol, sedatives and tranquilizers may increase drowsiness. Be careful when driving a motor vehicle or operating machinery. Excitability may occur, especially in children. Do not use any other products containing diphenhydramine.
Pregnancy/breast-feeding warning
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
Adults and children 12 years and over: take 25 to 50 mg (1 to 2 capsule) every 4 to 6 hours; not more than 12 capsules in 24 hours.
Children 6 years to 12 years of age: take 25 mg (1 capsule) every 4 to 6 hours; not more than 6 capsules in 24 hours.
Children under 6 years of age: ask a doctor
Other information
Store at controlled room temperature 59-86 degrees F.
Inactive ingredients
Lactose, starch, gelatin with bisulfites and artificial colors.
Questions?
Adverse drug event call: (800) 616-2471
Principal display panel

Diphenhydramine Hcl 25 mg Caps
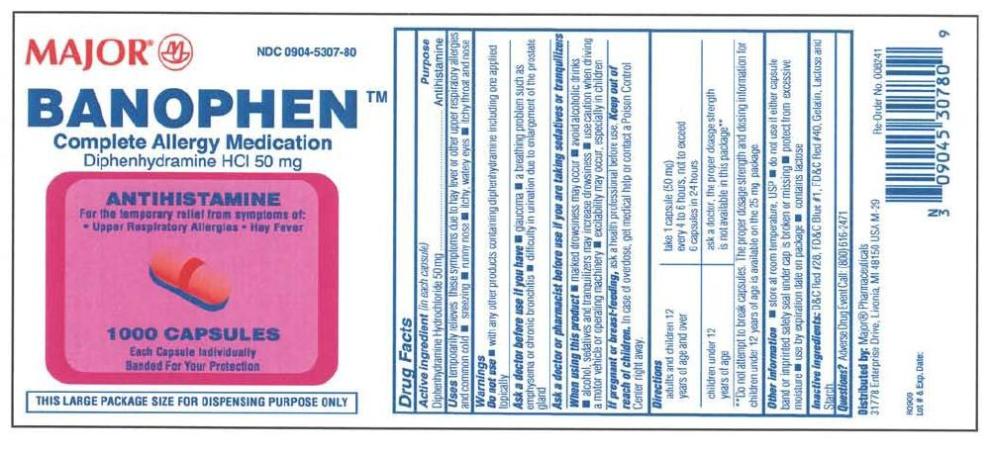
Active ingredient
Diphenhydramine Hydrochloride 50 mg
Purpose
Antihistamine
Use
Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies and common cold, sneezing, runny nose, itchy, watery eyes, itchy throat and nose.
Warning
Ask a doctor before use if you have glucoma, a breathing problem such as emphysema or chronic bronchitis, trouble urinating due to an enlarged prostate gland.
Ask a doctor before use if you have
glaucoma, a breathing problem such as emphysema or chronic bronchitis, trouble urinating due to an enlarged prostate gland.
Ask a doctor or pharmacist before use if you are
taking tranquilizers or sedative.
When using this product
you may get drowsy, avoid alcoholic drinks. Alcohol, sedatives, and tranquilizers may increase drowsiness. Be careful when driving a motor vehicle or operating machinery. Excitability may occur, especially in children. Do not use any other products containing diphenhydramine.
Pregnancy/breast-feeding warning
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children
Keep out of reach of children. In case of overdose, get medical help or contact Poison Control Center right away.
Directions
Adults and children 12 years and over: take 50 mg (1 capsule) every 4 to 6 hours; not more than 6 capsules in 24 hours.
Children under 12 years: ask a doctor, the proper dosage strength is not available in this package**
Do not attempt to break capsules. The proper dosage strength and dosing information for childrent under 12 years of age is available on the 25 mg package.
Other information
Store at controlled room temperature (59-86 degrees F). Protect from excessive moisture.
Inactive ingredients
Lactose, starch, gelatin with bisulfites and artificial colors
Questions?
Adverse drug event call: (800) 616-2471
Principal display panel

Diphenhydramine Hcl 50 mg Caps
| DIPHENHYDRAMINE HYDROCHLORIDE
diphenhydramine hydrochloride capsule |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH FINAL | part348 | 01/02/2009 | |
| DIPHENHYDRAMINE HYDROCHLORIDE
diphenhydramine hydrochloride capsule |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH FINAL | part348 | 01/02/2009 | |
| BANOPHEN
diphenhydramine hydrochloride capsule |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH FINAL | part348 | 01/02/2009 | |
| Labeler - Major Pharmaceuticals (191427277) |