DEXTROSE MONOHYDRATE
-
dextrose monohydrate injection
Amphastar Pharmaceuticals, Inc.
----------
DEXTROSE INJECTION, USPDESCRIPTION
Dextrose is α-D (+) Glucopyranose, C6H12O6, a sugar usually obtained by the hydrolysis of starch. It has the following structural formula:
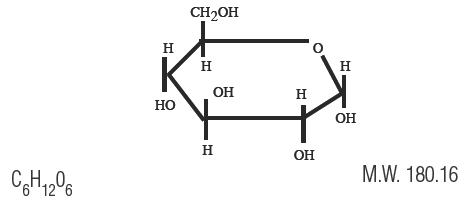
Dextrose, USP, contains one molecule of water of hydration or is anhydrous.
Dextrose Injection, USP, a fluid and nutrient replenisher, is a sterile aqueous solution of dextrose and is available in two concentrations:
- 25% - containing 250 mg of dextrose monohydrate per mL; osmolarity (calc.): 1250 mOsmol/ L.
- 50% - containing 500 mg of dextrose monohydrate per mL; osmolarity (calc.): 2500 mOsmol/ L.
These preparations are for intravenous use and contain no antimicrobial preservatives. Each is intended as a single dose vial; once the unit is assembled and used, any remaining portion of the solution must be discarded with the entire unit.
CLINICAL PHARMACOLOGY
Dextrose, the natural sugar occurring in blood, is the principal source of energy for the body. In addition to its importance as the primary source of energy for the body, dextrose has a multitude of other essential roles in the body economy. It is readily converted to fat, which provides a rich store of energy in a concentrated form (9 cal/g). Dextrose is also stored within the liver and muscles as glycogen. When a rapid rise in blood sugar is demanded by the body, glycogen is quickly liberated as D-glucose. When the supply of dextrose is insufficient, the body mobilizes its fat stores, which are converted to acetate with production of energy by the same oxidative pathways employed in the combustion of the dextrose.
Another important use of dextrose in the total body economy is the sparing of proteins, which, in the absence of dextrose, may be deaminated to provide carbon moieties from their constituent amino acids. These deaminated fragments may undergo oxidation in order to release energy. Dextrose is also the probable source of glucuronic acid, with which many foreign substances and their metabolites combine to form excretion products. It probably provides the basic substances required for the formation of hyaluronates and chondroitin sulfates, the supporting structure of the organism. It can be converted to a pentose essential for the formation of nucleic acids by the cells.
INDICATIONS AND USAGE
Dextrose Injection, 25% and 50%, are used in parenteral hyperalimentation. They are hypertonic solutions and, when administered intravenously, cause cellular dehydration. They have been employed to promote diuresis by increasing the osmotic pressure of the glomerular filtrate. Its hypertonic property makes it valuable in the following special clinical uses, which may be summarized as follows:
- a)
- For its concentrated food value in patients in whom more dilute solutions are contraindicated by actual or impending edema, such as exist in surgical as well as nonsurgical patients.
- b)
- 25%: Acute symptomatic episodes of hypoglycemia in the neonate or older infant to restore depressed blood glucose levels and control symptoms.
- c)
- 50%: Used in the treatment of insulin hypoglycemia (hyperinsulinemia or insulin shock) to restore blood glucose levels.
For reduction of increased cerebrospinal pressure and/ or cerebral edema due to delirium tremens or acute alcoholic intoxication. Increased cerebrospinal fluid pressure may be depressed for two to four hours after intravenous injection of 50 mL of 50% dextrose solution.
CONTRAINDICATIONS
Hypertonic solutions of dextrose should not be administered subcutaneously, intramuscularly, or intraperitoneally.
Subcutaneous administration of hypertonic solutions may be irritating to the tissues and should be avoided.
The intravenous use of strongly hypertonic solutions of dextrose to reduce cerebral edema and/or cerebrospinal pressure is contraindicated in the presence of intracranial or intraspinal hemorrhage and in delirium tremens if the patient is already dehydrated. It is also contraindicated in diabetic coma while blood sugar is excessively high and in patients with glucose-galactose malabsorption syndrome.
WARNINGS
Dextrose solutions I.V. can cause fluid or solute overload resulting in dilution of serum electrolyte concentrations, overhydration, congested states, or pulmonary edema.
Hypertonic dextrose solutions, even when given intravenously, have a tendency to cause venous thrombosis. Proper technique for intravenous injection should therefore be employed to avoid vein damage. For example: It is suggested that a needle of the smallest practicable bore be used; that the injection be made as slowly as conditions permit; that the bevel of the inserted needle be kept as far away as possible from the wall of the vein (usually the superior vena cava or other equally large vein); blood flow in the punctured vein be increased by the application of heat to that extremity; that the solution itself be warmed to body temperature, or at least to room temperature, before use; and that the tourniquet be removed as soon as the needle is in the vein and before any solution is injected.
Dextrose-containing solutions should be used with caution in patients with subclinical or overt diabetes mellitus or carbohydrate intolerance.
Rapid administration of hypertonic solutions may produce significant hyperglycemia or hyperosmolar syndrome, especially in patients with chronic uremia or carbohydrate intolerance.
PRECAUTIONS
General
When using concentrated solutions of dextrose, it is important that these should be injected very slowly so as not to cause a local rise in the osmotic tension of the blood at the point of injection and administered so that extravasation does not occur. If thrombosis occurs during administration, the injection should be stopped and corrective measures instituted.
The use of hypertonic solutions requires particular care. Frequent observation of the tongue and determination of the ratio of red blood cells to plasma (hematocrit) are useful guides. Clinical evaluations and laboratory determinations should be performed to monitor fluid balance, electrolyte concentrations and acid-base balance.
The intravenous use of strongly hypertonic solutions of dextrose to reduces cerebral edema and/or cerebrospinal pressure requires very special care. It is significant to note that, following the initial fall in intracranial pressure, there is often a secondary rise, which may necessitate repeated spinal punctures or additional injections of the dextrose injection.
Hyperglycemia and glycosuria may be functions of the rate of administration of metabolic insufficiency. To minimize these conditions, the infusion rate should be slowed down and the blood and urine glucose monitored. If necessary, insulin may be administered. When concentrated dextrose infusion is abruptly withdrawn, administer 5% or 10% dextrose to avoid reactive hypoglycemia.
Potassium
Excessive administration of potassium free solutions may result in significant hypokalemia. Potassium should be added to dextrose solutions and administered to fasting patients with good renal function, especially those on digitalis therapy.
Drug Interactions
Parenteral dextrose solutions should be cautiously administered, especially those containing sodium ions, to patients receiving corticosteroids or corticotropin.
Dextrose administration may cause vitamin B complex deficiency.
Additives may be incompatible. When introducing additives, use aseptic technique, mix thoroughly and do not store. Dextrose should not be administered simultaneously with blood through the same infusion set because pseudoagglutination of red blood cells may occur.
Usage in Pregnancy
Teratogenic Effect
Pregnancy Category C
Safety for use during pregnancy has not been established. Dextrose should be used only when clearly needed and when the potential benefits outweigh the unknown potential hazards to the fetus.
Usage in Children
Dextrose should be used with caution in infants of diabetic mothers, except as may be indicated in hypoglycemic neonates.
ADVERSE REACTIONS
A number of serious reactions have been reported following the intravenous injection of dextrose, which can be attributed to the solution or error in technique. These include: febrile response; infection at the injection site; tissue necrosis; venous thrombosis or phlebitis extending from the site of injection; extravasation; hypovolemia; hypervolemia; dehydration; mental confusion or unconsciousness. It has been claimed that dextrose may produce allergic reactions in corn-sensite persons, but this allegation has been refuted. The largest available peripheral vein and a well placed small bore needle should be used.
Thrombophlebitis may result from the use of hypertonic solutions via the intravenous route of administration. Rapid infusion (e.g., 50 to 100 g over three minutes, as in an intravenous glucose tolerance test) may occasionally cause a generalized flush. This subsides within ten minutes. Significant hyperglycemia, hyperosmolar syndrome and glycosuria may occur with too rapid administration of hypertonic solutions (See WARNINGS). They are more likely to cause irritation and should therefore be administered into larger central veins.
The administration of dextrose without an adequate provision of certain B vitamins, which form the coenzyme systems in its metabolism, will exhaust tissue stores of these factors, leading to deficiency states. Similarly, the utilization of glucose will cause the intracellular movement of potassium, so that provisions for replacement of this element during periods of dextrose administration may require consideration under certain conditions. The level of blood sugar appears to be one factor in governing the rate of production of certain hormones; hyperglycemia invokes an outpouring of insulin; hypoglycemia causes release of epinephrine and through this latter mechanism, the pituitary-adrenal cortex is stimulated. From the anterior pituitary and adrenal cortex, the anti-insulin factors which tend to restore the blood sugar to normal are liberated.
OVERDOSAGE
In the event of a fluid or solute overload during parenteral therapy, the patient's condition should be reevaluated and appropriate corrective treatment instituted.
DOSAGE AND ADMINISTRATION
Please note that the optional STICK-GARD® Safety Needle, featured with stock number 2001 is interchangeable with a standard needle.
Hypertonic solutions of dextrose are for intravenous use only.
The concentration and dose depend upon the patient's age, weight and clinical condition. Electrolytes should be added based on fluid and electrolyte status.
The maximum rate at which dextrose can be infused without producing glycosuria is 0.5 g/kg/hour. About 95% is retained when infused at 0.8 g/kg/hour.
Insulin-induced hypoglycemia
Blood glucose is determined before injecting dextrose. In emergencies, dextrose is promptly administered without waiting for pretreatment test results.
- Adults: 10 to 25 g (20 to 50 mL of the 50% solution). Repeated doses may be required in severe cases. The number of injections and the interval between them must be determined in each case by clinical judgment. Slow intravenous administration is recommended, e.g. 3 mL of the 50% solution or 6 mL of the 25% solution per minute. After 25 grams of dextrose have been given, it is advisable to interrupt the injection and evaluate the effect. The exact number of grams required to relieve hypoglycemia will vary. After the patient responds, supplemental oral feedings are indicated to avoid relapse, especially after insulin shock therapy.
- Neonates: 250 to 500 mg/kg/dose (5 to 10 mL of 25% dextrose in a 5 kg infant) to control acute symptomatic hypoglycemia.
- Severe cases or older infants: Larger or repeated single doses up to 10 to 12 mL of 25% dextrose may be required. Subsequent continuous I.V. infusion of 10% dextrose may be needed to stabilize blood glucose levels.
In the treatment of acute alcoholism, intravenous dextrose - 50 mL of the 50% solution - is used; unmodified insulin, 20 units, and thiamine hydrochloride, 100 mg, are added to the infusion.
NOTE: Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
HOW SUPPLIED
In unit-use packages containing a MIN-I-JET® disposable syringe.
DEXTROSE INJECTION, USP, 50%
| Stock No. 1001 (with 18 G. × 1-1/2" needle) | NDC 0548-1001-00 | 25 g in 50 mL |
In unit-use packages containing a disposable MIN-I-JET® Luer-Lock syringe with optional STICK-GARD® Safety Needle.
DEXTROSE INJECTION, USP, 50%
| Stock No. 2001 | NDC 0548-2001-00 | 25 g in 50 mL |
Twenty-five unit-use packages per carton.
Manufactured under U.S. Pat. No. 4,834, 716, Reissue No. 33,617, STICK-GARD® Safety Needle.
In unit-use packages containing a Luer-Jet™ Luer-Lock Prefilled Syringe.
DEXTROSE INJECTION, USP, 25%
| Stock No. 3315 | NDC 0548-3315-00 | 2.5 g in 10 mL |
DEXTROSE INJECTION, USP, 50%
| Stock No. 3301 | NDC 0548-3301-00 | 25 g in 50 mL |
Ten unit-use packages per carton.
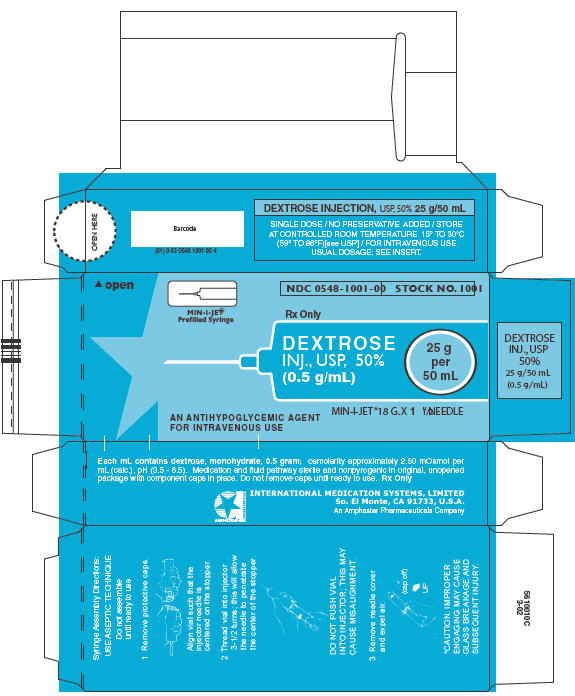
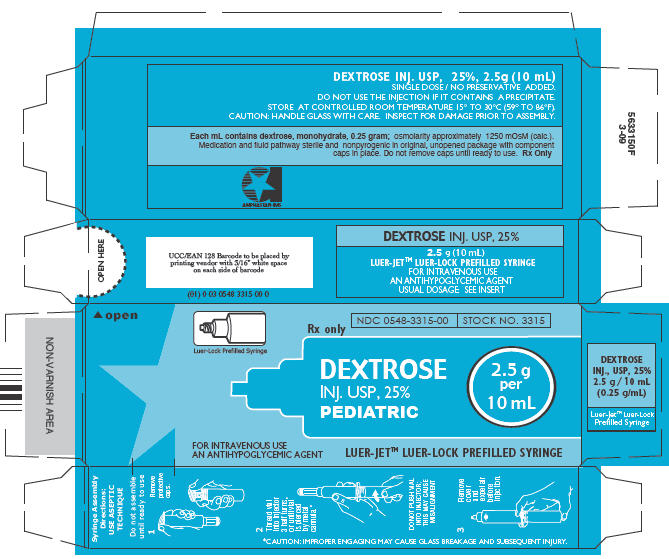
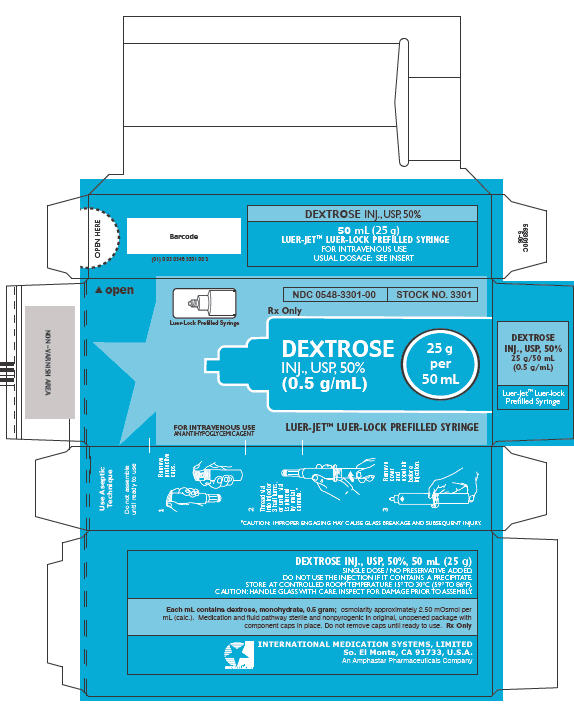
Syringe Assembly Directions
The MIN-I-JET® syringe with needle, illustrated below, is the basic unit upon which all the other syringe systems are built; slight adaptations and /or additional auxillary parts create the other syringe systems. Assembly directions remain essentially the same.
USE ASEPTIC TECHNIQUE
Do not assemble until ready to use.
|
||
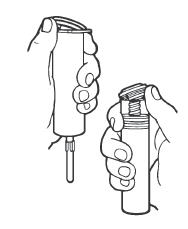 | 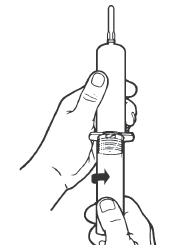 | 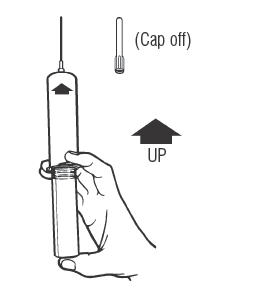 |
| Remove protective caps. Align vial such that the injector needle is centered on the stopper. | Thread vial into injector 3 half turns, or until needle penetrates stopper.* DO NOT PUSH VIAL INTO INJECTOR; THIS MAY CAUSE MISALIGNMENT. | Remove needle cap and expel air. |
Store at controlled room temperature 15° to 30°C (59° to 86°F).
INTERNATIONAL MEDICATION SYSTEMS, LIMITED
SO. EL MONTE, CA 91733, U.S.A.
An Amphastar Pharmaceuticals Company
Rx Only
REV. 1-08
6910010J
PRINCIPAL DISPLAY PANEL - MIN-I-JET® Prefilled Syringe Carton
MIN-I-JET®
Prefilled Syringe
NDC 0548-1001-00
STOCK NO. 1001
Rx Only
DEXTROSE
INJ., USP, 50%
(0.5 g/mL)
25 g
per
50 mL
AN ANTIHYPOGLYCEMIC AGENT
FOR INTRAVENOUS USE
MIN-I-JET® 18 G. X 1 " 1/2 NEEDLE
PRINCIPAL DISPLAY PANEL - STICK-GARD® Prefilled Syringe Carton
FEATURING STICK-GARD®
18 G. SAFETY INJECTOR
NDC 0548-2001-00
STOCK NO. 2001
Rx Only
DEXTROSE
INJ., USP, 50%
(0.5 g/mL)
25 g
per
50 mL
AN ANTIHYPOGLYCEMIC AGENT
FOR INTRAVENOUS USE
STICK-GARD® PREFILLED SYRINGE

PRINCIPAL DISPLAY PANEL -LUER-JET™ Prefilled Syringe Pediatric Carton
Luer-Lock Prefilled Syringe
NDC 0548-3315-00
STOCK NO. 3315
Rx only
DEXTROSE
INJ. USP, 25%
PEDIATRIC
2.5 g
per
10 mL
FOR INTRAVENOUS USE
AN ANTIHYPOGLYCEMIC AGENT
LUER-JET™ LUER-LOCK PREFILLED SYRINGE
PRINCIPAL DISPLAY PANEL - LUER-JET Prefilled Syringe Carton
Luer-Lock Prefilled Syringe
NDC 0548-3301-00
STOCK NO. 3301
Rx Only
DEXTROSE
INJ., USP, 50%
(0.5 g/mL)
25 g
per
50 mL
FOR INTRAVENOUS USE
AN ANTIHYPOGLYCEMIC AGENT
LUER-JET™ LUER-LOCK PREFILLED SYRINGE
| DEXTROSE MONOHYDRATE
dextrose monohydrate injection |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| UNAPPROVED DRUG OTHER | 06/01/2000 | 12/31/2009 | |
| DEXTROSE MONOHYDRATE
dextrose monohydrate injection |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| UNAPPROVED DRUG OTHER | 01/02/1973 | ||
| DEXTROSE MONOHYDRATE
dextrose monohydrate injection |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| UNAPPROVED DRUG OTHER | 12/01/1989 | 12/31/2003 | |
| DEXTROSE MONOHYDRATE
dextrose monohydrate injection |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| UNAPPROVED DRUG OTHER | 06/01/2000 | 12/31/2003 | |
| Labeler - Amphastar Pharmaceuticals, Inc. (024736733) |