desoxyn (Methamphetamine Hydrochloride) tablet
[OVATION Pharmaceuticals, Inc.]
C II
METHAMPHETAMINE HAS A HIGH POTENTIAL FOR ABUSE. IT SHOULD THUS BE TRIED ONLY IN WEIGHT REDUCTION PROGRAMS FOR PATIENTS IN WHOM ALTERNATIVE THERAPY HAS BEEN INEFFECTIVE. ADMINISTRATION OF METHAMPHETAMINE FOR PROLONGED PERIODS OF TIME IN OBESITY MAY LEAD TO DRUG DEPENDENCE AND MUST BE AVOIDED. PARTICULAR ATTENTION SHOULD BE PAID TO THE POSSIBILITY OF SUBJECTS OBTAINING METHAMPHETAMINE FOR NON-THERAPEUTIC USE OR DISTRIBUTION TO OTHERS, AND THE DRUG SHOULD BE PRESCRIBED OR DISPENSED SPARINGLY.
DESCRIPTION
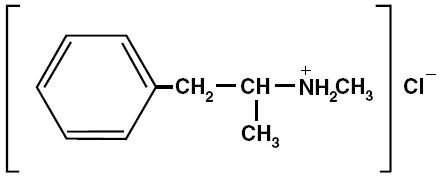
DESOXYN (methamphetamine hydrochloride tablets, USP), chemically known as (S)-N, α-dimethylbenzeneethanamine hydrochloride, is a member of the amphetamine group of sympathomimetic amines. It has the following structural formula:
DESOXYN tablets contain 5 mg of methamphetamine hydrochloride for oral administration.
Inactive Ingredients
Corn starch, lactose, sodium paraminobenzoate, stearic acid and talc.
CLINICAL PHARMACOLOGY
Methamphetamine is a sympathomimetic amine with CNS stimulant activity. Peripheral actions include elevation of systolic and diastolic blood pressures and weak bronchodilator and respiratory stimulant action. Drugs of this class used in obesity are commonly known as "anorectics" or "anorexigenics". It has not been established, however, that the action of such drugs in treating obesity is primarily one of appetite suppression. Other central nervous system actions, or metabolic effects, may be involved, for example.
Adult obese subjects instructed in dietary management and treated with "anorectic" drugs, lose more weight on the average than those treated with placebo and diet, as determined in relatively short-term clinical trials.
The magnitude of increased weight loss of drug-treated patients over placebo-treated patients is only a fraction of a pound a week. The rate of weight loss is greatest in the first weeks of therapy for both drug and placebo subjects and tends to decrease in succeeding weeks. The origins of the increased weight loss due to the various possible drug effects are not established. The amount of weight loss associated with the use of an "anorectic" drug varies from trial to trial, and the increased weight loss appears to be related in part to variables other than the drug prescribed, such as the physician-investigator, the population treated, and the diet prescribed. Studies do not permit conclusions as to the relative importance of the drug and non-drug factors on weight loss.
The natural history of obesity is measured in years, whereas the studies cited are restricted to a few weeks duration; thus, the total impact of drug-induced weight loss over that of diet alone must be considered clinically limited.
The mechanism of action involved in producing the beneficial behavioral changes seen in hyperkinetic children receiving methamphetamine is unknown.
In humans, methamphetamine is rapidly absorbed from the gastrointestinal tract. The primary site of metabolism is in the liver by aromatic hydroxylation, N-dealkylation and deamination. At least seven metabolites have been identified in the urine. The biological half-life has been reported in the range of 4 to 5 hours. Excretion occurs primarily in the urine and is dependent on urine pH. Alkaline urine will significantly increase the drug half-life. Approximately 62% of an oral dose is eliminated in the urine within the first 24 hours with about one-third as intact drug and the remainder as metabolites.
INDICATIONS AND USAGE
Attention Deficit Disorder with Hyperactivity
DESOXYN tablets are indicated as an integral part of a total treatment program which typically includes other remedial measures (psychological, educational, social) for a stabilizing effect in children over 6 years of age with a behavioral syndrome characterized by the following group of developmentally inappropriate symptoms: moderate to severe distractibility, short attention span, hyperactivity, emotional lability, and impulsivity. The diagnosis of this syndrome should not be made with finality when these symptoms are only of comparatively recent origin. Nonlocalizing (soft) neurological signs, learning disability, and abnormal EEG may or may not be present, and a diagnosis of central nervous system dysfunction may or may not be warranted.
Exogenous Obesity
As a short-term (i.e., a few weeks) adjunct in a regimen of weight reduction based on caloric restriction, for patients in whom obesity is refractory to alternative therapy, e.g., repeated diets, group programs, and other drugs.
The limited usefulness of DESOXYN tablets (see CLINICAL PHARMACOLOGY) should be weighed against possible risks inherent in use of the drug, such as those described below.
CONTRAINDICATIONS
DESOXYN tablets are contraindicated during or within 14 days following the administration of monoamine oxidase inhibitors; hypertensive crisis may result. It is also contraindicated in patients with glaucoma, advanced arteriosclerosis, symptomatic cardiovascular disease, moderate to severe hypertension, hyperthyroidism or known hypersensitivity or idiosyncrasy to sympathomimetic amines. Methamphetamine should not be given to patients who are in an agitated state or who have a history of drug abuse.
WARNINGS
Tolerance to the anorectic effect usually develops within a few weeks. When this occurs, the recommended dose should not be exceeded in an attempt to increase the effect; rather, the drug should be discontinued (see DRUG ABUSE AND DEPENDENCE).
Decrements in the predicted growth (i.e., weight gain and/or height) rate have been reported with the long-term use of stimulants in children. Therefore, patients requiring long-term therapy should be carefully monitored.
Usage in Nursing Mothers
Amphetamines are excreted in human milk. Mothers taking amphetamines should be advised to refrain from nursing.
PRECAUTIONS
General
DESOXYN tablets should be used with caution in patients with even mild hypertension.
Methamphetamine should not be used to combat fatigue or to replace rest in normal persons.
Prescribing and dispensing of methamphetamine should be limited to the smallest amount that is feasible at one time in order to minimize the possibility of overdosage.
Information for Patients
The patient should be informed that methamphetamine may impair the ability to engage in potentially hazardous activities, such as, operating machinery or driving a motor vehicle.
The patient should be cautioned not to increase dosage, except on advice of the physician.
Drug Interactions
Insulin requirements in diabetes mellitus may be altered in association with the use of methamphetamine and the concomitant dietary regimen.
Methamphetamine may decrease the hypotensive effect of guanethidine.
DESOXYN should not be used concurrently with monoamine oxidase inhibitors (see CONTRAINDICATIONS).
Concurrent administration of tricyclic antidepressants and indirect-acting sympathomimetic amines such as the amphetamines, should be closely supervised and dosage carefully adjusted.
Phenothiazines are reported in the literature to antagonize the CNS stimulant action of the amphetamines.
Drug/Laboratory Test Interactions
Literature reports suggest that amphetamines may be associated with significant elevation of plasma corticosteroids. This should be considered if determination of plasma corticosteroid levels is desired in a person receiving amphetamines.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Data are not available on long-term potential for carcinogenicity, mutagenicity, or impairment of fertility.
Pregnancy
Teratogenic Effects
Pregnancy Category C. Methamphetamine has been shown to have teratogenic and embryocidal effects in mammals given high multiples of the human dose. There are no adequate and well-controlled studies in pregnant women. DESOXYN tablets should not be used during pregnancy unless the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects
Infants born to mothers dependent on amphetamines have an increased risk of premature delivery and low birth weight. Also, these infants may experience symptoms of withdrawal as demonstrated by dysphoria, including agitation and significant lassitude.
Nursing Mothers
See WARNINGS.
Pediatric Use
Safety and effectiveness for use as an anorectic agent in children below the age of 12 years have not been established.
Long-term effects of methamphetamine in children have not been established (see WARNINGS).
Drug treatment is not indicated in all cases of the behavioral syndrome characterized by moderate to severe distractibility, short attention span, hyperactivity, emotional lability and impulsivity. It should be considered only in light of the complete history and evaluation of the child. The decision to prescribe DESOXYN tablets should depend on the physician's assessment of the chronicity and severity of the child's symptoms and their appropriateness for his/her age. Prescription should not depend solely on the presence of one or more of the behavioral characteristics.
When these symptoms are associated with acute stress reactions, treatment with DESOXYN tablets is usually not indicated.
Clinical experience suggests that in psychotic children, administration of DESOXYN tablets may exacerbate symptoms of behavior disturbance and thought disorder.
Amphetamines have been reported to exacerbate motor and phonic tics and Tourette's syndrome. Therefore, clinical evaluation for tics and Tourette's syndrome in children and their families should precede use of stimulant medications.
Geriatric Use
Clinical Studies of DESOXYN did not include sufficient numbers of subjects age 65 years and over to determine whether elderly subjects respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy observed in this population.
ADVERSE REACTIONS
The following are adverse reactions in decreasing order of severity within each category that have been reported:
Cardiovascular: Elevation of blood pressure, tachycardia and palpitation. Fatal cardiorespiratory arrest has been reported, mostly in the context of abuse/misuse.
Central Nervous System: Psychotic episodes have been rarely reported at recommended doses. Dizziness, dysphoria, overstimulation, euphoria, insomnia, tremor, restlessness and headache. Exacerbation of motor and phonic tics and Tourette's syndrome.
Gastrointestinal: Diarrhea, constipation, dryness of mouth, unpleasant taste and other gastrointestinal disturbances.
Hypersensitivity: Urticaria.
Endocrine: Impotence and changes in libido.
Miscellaneous: Suppression of growth has been reported with the long-term use of stimulants in children (see WARNINGS).
DRUG ABUSE AND DEPENDENCE
Controlled Substance
DESOXYN tablets are subject to control under DEA schedule II.
Abuse
Methamphetamine has been extensively abused. Tolerance, extreme psychological dependence, and severe social disability have occurred. There are reports of patients who have increased the dosage to many times that recommended. Abrupt cessation following prolonged high dosage administration results in extreme fatigue and mental depression; changes are also noted on the sleep EEG. Manifestations of chronic intoxication with methamphetamine include severe dermatoses, marked insomnia, irritability, hyperactivity, and personality changes. The most severe manifestation of chronic intoxication is psychosis often clinically indistinguishable from schizophrenia. Abuse and/or misuse of methamphetamine have resulted in death. Fatal cardiorespiratory arrest has been reported in the context of abuse and/or misuse of methamphetamine.
OVERDOSAGE
Manifestations of acute overdosage with methamphetamine include restlessness, tremor, hyperreflexia, rapid respiration, confusion, assaultiveness, hallucinations, panic states, hyperpyrexia, and rhabdomyolysis. Fatigue and depression usually follow the central stimulation. Cardiovascular effects include arrhythmias, hypertension or hypotension, and circulatory collapse. Gastrointestinal symptoms include nausea, vomiting, diarrhea, and abdominal cramps. Fatal poisoning usually terminates in convulsions and coma.
Consult with a Certified Poison Control Center regarding treatment for up to date guidance and advice. Management of acute methamphetamine intoxication is largely symptomatic and includes gastric evacuation, administration of activated charcoal, and sedation. Experience with hemodialysis or peritoneal dialysis is inadequate to permit recommendations in this regard.
Acidification of urine increases methamphetamine excretion, but is believed to increase risk of acute renal failure if myoglobinuria is present. Intravenous phentolamine (Regitine®) has been suggested for possible acute, severe hypertension, if this complicates methamphetamine overdosage. Usually a gradual drop in blood pressure will result when sufficient sedation has been achieved. Chlorpromazine has been reported to be useful in decreasing CNS stimulation and sympathomimetic effects.
DOSAGE AND ADMINISTRATION
DESOXYN tablets are given orally.
Methamphetamine should be administered at the lowest effective dosage, and dosage should be individually adjusted. Late evening medication should be avoided because of the resulting insomnia.
Attention Deficit Disorder with Hyperactivity: For treatment of children 6 years or older with a behavioral syndrome characterized by moderate to severe distractibility, short attention span, hyperactivity, emotional lability and impulsivity: An initial dose of 5 mg DESOXYN once or twice a day is recommended. Daily dosage may be raised in increments of 5 mg at weekly intervals until an optimum clinical response is achieved. The usual effective dose is 20 to 25 mg daily. The total daily dose may be given in two divided doses daily.
Where possible, drug administration should be interrupted occasionally to determine if there is a recurrence of behavioral symptoms sufficient to require continued therapy.
For Obesity: One 5 mg tablet should be taken one-half hour before each meal. Treatment should not exceed a few weeks in duration. Methamphetamine is not recommended for use as an anorectic agent in children under 12 years of age.
HOW SUPPLIED
DESOXYN (methamphetamine hydrochloride tablets, USP) is supplied as white tablets imprinted with the letters OV on one side and the number 12 on the opposite side, containing 5 mg methamphetamine hydrochloride in bottles of 100 (NDC 67386-102-01).
Recommended Storage: Store below 86°F (30°C).
Dispense in a USP tight, light resistant container.
Rev: November, 2006
Manufactured by:
Abbott Pharmaceuticals PR Ltd.
Barceloneta, PR 00617
For:
OVATION Pharmaceuticals, Inc.
Deerfield, IL 60015, U.S.A.
| Desoxyn (Methamphetamine Hydrochloride) | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
Revised: 11/2006OVATION Pharmaceuticals, Inc.