econochlor (chloramphenicol) solution
econochlor (chloramphenicol) ointment
[Alcon Laboratories, Inc.]
Bone marrow hypoplasia, including aplastic anemia and death has been reported following local application of chloramphenicol. Chloramphenicol should not be used when less potentially dangerous agents would be expected to provide effective treatment.
DESCRIPTION
ECONOCHLOR® (Chloramphenicol) is a sterile topical ophthalmic antibacterial prepared in solution and ointment forms. The active ingredient is represented by the chemical structure:
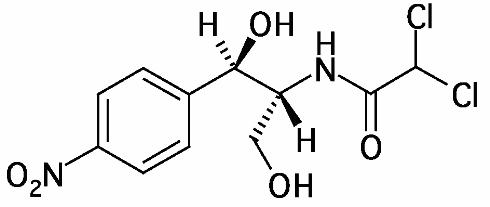
Established name:
Chloramphenicol
Chemical name:
Acetamide, 2,2-dichloro-N-[2-hydroxy-1-(hydroxymethyl)-2- (4-nitrophenyl) ethyl]-, [R-(R*,R)]-
Each ml of solution contains: Active: Chloramphenicol 0.5% (5mg/ml). Preservative: Thimerosal 0.01%. Vehicle: Hydroxypropyl Methylcellulose: Inactive: Boric Acid, Sodium Borate (to adjust pH), Purified Water.
DM-00
Each gram of ointment contains: Active: Chloramphenicol 1.0% (10 mg/g). Inactive: Mineral Oil, Anhydrous Liquid Lanolin, White Petrolatum.
DM-00
CLINICAL PHARMACOLOGY
Chloramphenicol is a broad-spectrum antibiotic originally isolated from Streptomyces venezuelae. It is primarily bacteriostatic and acts by inhibition of protein synthesis by interfering with the transfer of activated amino acids from soluble RNA to ribosomes. Development of resistance to chloramphenicol can be regarded as minimal for staphylococci and many other species of bacteria.
INDICATIONS USAGE
Chloramphenicol should be used only in those serious infections for which less potentially dangerous drugs are ineffective or contraindicated. Bacteriological studies should be performed to determine the causative organisms and their sensitivity to chloramphenicol (See Box Warning). For treatment of ocular infections involving the conjunctiva and/or cornea caused by chloramphenicol-susceptible organisms.
Chloramphenicol has a wide spectrum of antimicrobial activity and is effective against many gram negative and gram positive bacteria, including the following common eye pathogens:
Staphylococcus aureus
Streptococci, including Streptococcus pneumoniae
Escherichia coli
Haemophilus influenzae
Klebsiella/Enterobacter species
Neisseria species
Moraxella lacunata (Morax-Axenfeld bacillus)
The product does not provide adequate coverage against:
Pseudomonas aeruginosa
Serratia marcescens
CONTRAINDICATIONS
This product is contraindicated in those persons who have shown hypersensitivity to any of its components.
WARNINGS
SEE BOX WARNING.
Ophthalmic ointment may retard corneal wound healing.
PRECAUTIONS
Prolonged use of antibiotics may occasionally result in overgrowth of non-susceptible organisms, including fungi. If new infections appear during treatment, the therapy should be altered. In all serious infections, the topical use of chloramphenicol should be supplemented by appropriate systemic medication.
ADVERSE REACTIONS
Blood dyscrasias may be associated with the systemic use of chloramphenicol. One case of bone-marrow hypoplasia following the prolonged (23 month) topical use of chloramphenicol ophthalmic solution has been reported.
DOSAGE ADMINISTRATION
Solution: In severe disease, instill two drops on the eye(s) hourly until improvement, following which treatment should be diminished prior to discontinuation. In mild disease, drops may be used four times daily. Ointment: Apply a small amount to the lower conjunctival sac(s) at bedtime as a supplement to the drops.
HOW SUPPLIED
Solution in 5ml (2.5ml fill) and 15ml plastic Drop-Tainer® dispensers. Ointment in 3.5 gram ophthalmic tube. Rx only.
2.5ml NDC 0065-0642-25
15ml NDC 0065-0642-15
3.5 g NDC 0065-0641-35
STORAGE
Solution: Refrigerate until dispensed. Ointment: Store between 46° and 80°F.
June, 1984
29824
Printed in USA
Alcon
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 USA
| Econochlor (chloramphenicol) | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Econochlor (chloramphenicol) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Revised: 09/2006Alcon Laboratories, Inc.