celontin (methsuximide) capsule
[Parke-Davis]
DESCRIPTION
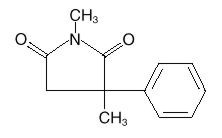
Celontin (methsuximide) is an anticonvulsant succinimide, chemically designated as N,2-Dimethyl-2-phenylsuccinimide, with the following structural formula:
Each Celontin capsule contains 150 mg or 300 mg methsuximide, USP. Also contains starch, NF. The capsule contains colloidal silicon dioxide, NF; D&C yellow No. 10; FD&C yellow No. 6; gelatin, NF; and sodium lauryl sulfate, NF.
CLINICAL PHARMACOLOGY
Methsuximide suppresses the paroxysmal three cycle per second spike and wave activity associated with lapses of consciousness which is common in absence (petit mal) seizures. The frequency of epileptiform attacks is reduced, apparently by depression of the motor cortex and elevation of the threshold of the central nervous system to convulsive stimuli.
INDICATIONS AND USAGE
Celontin is indicated for the control of absence (petit mal) seizures that are refractory to other drugs.
CONTRAINDICATIONS
Methsuximide should not be used in patients with a history of hypersensitivity to succinimides.
WARNINGS
Blood dyscrasias, including some with fatal outcome, have been reported to be associated with the use of succinimides; therefore, periodic blood counts should be performed. Should signs and/or symptoms of infection (eg, sore throat, fever) develop, blood counts should be considered at that point.
It has been reported that succinimides have produced morphological and functional changes in animal liver. For this reason, methsuximide should be administered with extreme caution to patients with known liver or renal disease. Periodic urinalysis and liver function studies are advised for all patients receiving the drug.
Cases of systemic lupus erythematosus have been reported with the use of succinimides. The physician should be alert to this possibility.
Usage in Pregnancy
Reports suggest an association between the use of anticonvulsant drugs by women with epilepsy and an elevated incidence of birth defects in children born to these women. Data are more extensive with respect to phenytoin and phenobarbital, but these are also the most commonly prescribed anticonvulsants; less systematic or anecdotal reports suggest a possible similar association with the use of all known anticonvulsant drugs.
The reports suggesting an elevated incidence of birth defects in children of drug-treated epileptic women cannot be regarded as adequate to prove a definite cause and effect relationship. There are intrinsic methodologic problems in obtaining adequate data on drug teratogenicity in humans; the possibility also exists that other factors, eg, genetic factors or the epileptic condition itself, may be more important than drug therapy in leading to birth defects. The great majority of mothers on anticonvulsant medication deliver normal infants. It is important to note that anticonvulsant drugs should not be discontinued in patients in whom the drug is administered to prevent major seizures because of the strong possibility of precipitating status epilepticus with attendant hypoxia and threat to life. In individual cases where the severity and frequency of the seizure disorder are such that the removal of medication does not pose a serious threat to the patient, discontinuation of the drug may be considered prior to and during pregnancy, although it cannot be said with any confidence that even minor seizures do not pose some hazard to the developing embryo or fetus.
The prescribing physician will wish to weigh these considerations in treating or counseling epileptic women of childbearing potential.
PRECAUTIONS
General
It is recommended that the physician withdraw the drug slowly on the appearance of unusual depression, aggressiveness, or other behavioral alterations.
As with other anticonvulsants, it is important to proceed slowly when increasing or decreasing dosage, as well as when adding or eliminating other medication. Abrupt withdrawal of anticonvulsant medication may precipitate absence (petit mal) status.
Methsuximide, when used alone in mixed types of epilepsy, may increase the frequency of grand mal seizures in some patients.
Information for Patients
Methsuximide may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a motor vehicle or other such activity requiring alertness; therefore, the patient should be cautioned accordingly. Patients taking methsuximide should be advised of the importance of adhering strictly to the prescribed dosage regimen.
Patients should be instructed to promptly contact their physician if they develop signs and/or symptoms suggesting an infection (eg, sore throat, fever).
ADVICE TO THE PHARMACIST AND PATIENT: Since methsuximide has a relatively low melting temperature (124° F), storage conditions which may promote high temperatures (closed cars, delivery vans, or storage near steam pipes) should be avoided. Do not dispense or use capsules that are not full or in which contents have melted. Effectiveness may be reduced. Protect from excessive heat (104° F).
Drug Interactions
Since Celontin (methsuximide) may interact with concurrently administered antiepileptic drugs, periodic serum level determinations of these drugs may be necessary (eg, methsuximide may increase the plasma concentrations of phenytoin and phenobarbital).
Pregnancy
See WARNINGS.
Pediatric Use
See DOSAGE AND ADMINISTRATION.
ADVERSE REACTIONS
Gastrointestinal System: Gastrointestinal symptoms occur frequently and have included nausea or vomiting, anorexia, diarrhea, weight loss, epigastric and abdominal pain, and constipation.
Hemopoietic System: Hemopoietic complications associated with the administration of methsuximide have included eosinophilia, leukopenia, monocytosis, and pancytopenia with or without bone marrow suppression.
Nervous System: Neurologic and sensory reactions reported during therapy with methsuximide have included drowsiness, ataxia or dizziness, irritability and nervousness, headache, blurred vision, photophobia, hiccups, and insomnia. Drowsiness, ataxia, and dizziness have been the most frequent side effects noted. Psychologic abnormalities have included confusion, instability, mental slowness, depression, hypochondriacal behavior, and aggressiveness. There have been rare reports of psychosis, suicidal behavior, and auditory hallucinations.
Integumentary System: Dermatologic manifestations which have occurred with the administration of methsuximide have included urticaria, Stevens-Johnson syndrome, and pruritic erythematous rashes.
Cardiovascular: Hyperemia.
Genitourinary System: Proteinuria, microscopic hematuria.
Body as a Whole: Periorbital edema.
OVERDOSAGE
Acute overdoses may produce nausea, vomiting, and CNS depression including coma with respiratory depression. Methsuximide poisoning may follow a biphasic course. Following an initial comatose state, patients have awakened and then relapsed into a coma within 24 hours. It is believed that an active metabolite of methsuximide, N-desmethylmethsuximide, is responsible for this biphasic profile. It is important to follow plasma levels of N-desmethylmethsuximide in methsuximide poisonings. Levels greater than 40 µg/mL have caused toxicity, and coma has been seen at levels of 150 µg/mL.
Treatment
Treatment should include emesis (unless the patient is or could rapidly become obtunded, comatose, or convulsing) or gastric lavage, activated charcoal, cathartics, and general supportive measures. Charcoal hemoperfusion may be useful in removing the N-desmethyl metabolite of methsuximide. Forced diuresis and exchange transfusions are ineffective.
DOSAGE AND ADMINISTRATION
Optimum dosage of Celontin must be determined by trial. A suggested dosage schedule is 300 mg per day for the first week. If required, dosage may be increased thereafter at weekly intervals by 300 mg per day for the three weeks following to a daily dosage of 1.2 g. Because therapeutic effect and tolerance vary among patients, therapy with Celontin must be individualized according to the response of each patient. Optimal dosage is that amount of Celontin which is barely sufficient to control seizures so that side effects may be kept to a minimum. The smaller capsule (150 mg) facilitates administration to small children.
Celontin may be administered in combination with other anticonvulsants when other forms of epilepsy coexist with absence (petit mal).
HOW SUPPLIED
N 0071-0525-24 (P-D 525)–Celontin Capsules, #1 capsule each containing 300 mg methsuximide; bottles of 100.
N 0071-0537-24 (P-D 537)–Celontin Capsules, Half-Strength, #3 capsule each containing 150 mg methsuximide; bottles of 100.
Store at 25°C (77°F); excursions permitted to 15–30°C (59–86°F) [see USP Controlled Room Temperature].
Protect from light and moisture. Protect from excessive heat 40°C (104°F).
Rx only

LAB-0156-2.0
October 2006
| Celontin (methsuximide) | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Celontin (methsuximide) | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
Revised: 11/2006