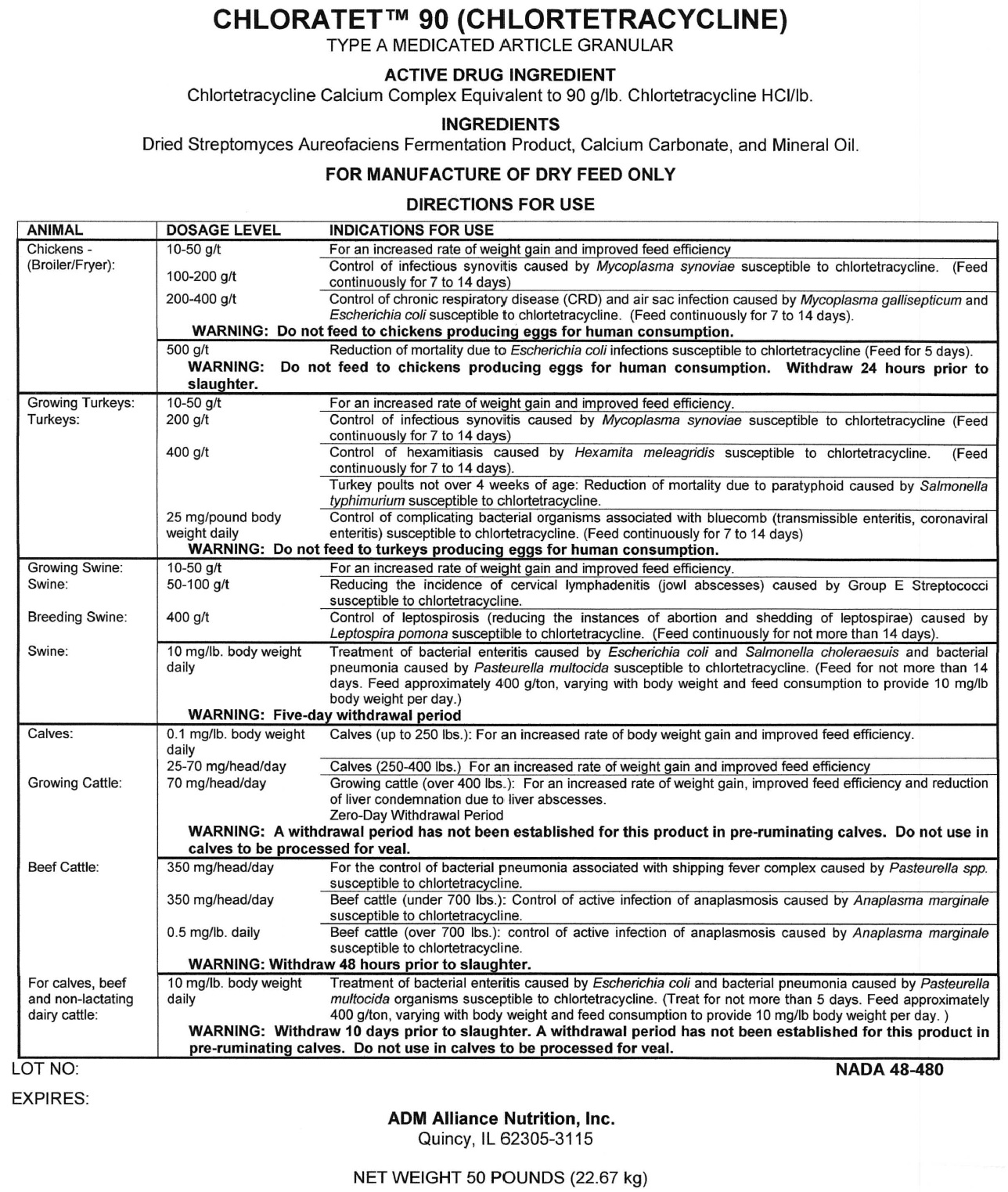
CHLORATET 90
-
chlortetracycline hydrochloride granule
ADM Alliance Nutrition, Inc
----------
CHLORTETRACYCLINE
TYPE A MEDICATED ARTICLE GRANULAR
ACTIVE DRUG INGREDIENTS
Chlortetracycline Calcium Complex Equivalent to 90 g/lb. Chlortetracycline HCl/lb.
INGREDIENTS
Dried Streptomyces Aureofaciens Fermentation Product, Calcium Carbonate, and Mineral Oil.
FOR MANUFACTURE OF DRY FEED ONLY
Directions for Use
Chicken - (Broiler/Fryer)
| DOSAGE LEVEL | INDICATION FOR USE |
| 10-50 g/t | For an increased rate of weight gain and improved feed efficiency |
| 100-200 g/t | Control
of infectious synovitis caused by Mycoplasma synoviae susceptible to
chlortetracycline. (Feed continuously for 7 to 14 days) |
| 200-400 g/t | Control
of chronic respiratory disease (CRD) and air sac infection caused by
Mycoplasma gallisepticum and Escherichia coli susceptible to
chlortetracycline. ( Feed continuously for 7 to 14 days) |
| DOSAGE LEVEL | INDICATION FOR USE |
| 500 g/t | Reduction of mortality due to Escherichia coli infections susceptible to chlortetracycline (Feed For 5 days) |
Growing Turkeys
| DOSAGE LEVEL | INDICATION FOR USE |
| 10-50 g/t | For an increased rate of weight gain and improved feed efficiency |
Turkeys
| DOSAGE LEVEL | INDICATION FOR USE |
| 200 g/t | Control
of infectious synovitis caused by Mycoplasma synoviae susceptible to
chlortetracycline. (Feed continuously for 7 to 14 days) |
| 400 g/t | Control of hexamitiasis caused by Hexamita meleagridis susceptible to chlortetracycline. (Feed continuously for 7 to 14 days) |
| 400 g/t | Turkey
poults not over 4 weeks of age: Reduction of mortality due to
paratyphoid caused by Salmonella typhimurium susceptible to
chlortetracycline. |
| 25 mg/pound body weight daily | Control
of complicating bacterial organisms associated with bluecomb
(transmissible enteritis, coronaviral enteritis) suseptible to
chlortetracycline. (Feed continuously for 7 to 14 days) |
Growing Swine
| DOSAGE LEVEL | INDICATION FOR USE |
| 10-50 g/t | For an increased rate of weight gain and improved feed efficiency |
Swine
| DOSAGE LEVEL | INDICATION FOR USE |
| 50-100 g/t | Reducing
the incidence of cervical lymphadenitis (jaw abscesses) caused by Group
E streptococci susceptible to chlortetracycline. |
Breeding Swine
| DOSAGE LEVEL | INDICATION FOR USE |
| 400 g/t | Control
of leptospirosis ( reducing the instances of abortion and shredding of
leptospirae) caused by Leptospira pomona susceptible to
chlortetracycline. (Feed continuously for not more than 14 days) |
Swine
| DOSAGE LEVEL | INDICATIONS FOR USE |
| 10 mg/lb. body weight daily | Treatment
of bacterial enteritis caused by Escherichia coli and Salmonella
choleraesuis and bacterial pneumonia caused by Pasteurella multocida
susceptible to chlortetracycline. ( Feed for not more than 14 days.
Feed approximately 400 g/ton, varying with body weight and feed
consumption to provide 10 mg/lb body weight per day.) |
Calves
| DOSAGE LEVEL | INDICATIONS FOR USE |
| 0.1 mg/lb. body weight daily | Calves (up to 250 lbs.): For an increased rate of body weight gain and improved feed efficiency. |
| 25-70 mg/head/day | Calves ( 250-400 lbs.) For an increased rate of weight gain and improved feed efficiency |
Growing Cattle
| DOSAGE LEVEL | INDICATION FOR USE |
| 70mg/head/day | Growing
cattle (over 400 lbs.): For an increased rate of weight gain, improved
feed efficiency and reduction of liver condemnation due to liver
abscesses. Zero-Day withdrawal period |
Beef Cattle
| DOSAGE LEVEL | INDICATION FOR USE |
| 350 mg/head/day | For
the control of bacterial pneumonia associated with shipping fever
complex caused by Pasteurella spp. susceptible to chlortetracycline. |
| 350 mg/head/day | Beef
cattle (under 700 lbs.): Control of active infection of anaplasmosis
caused by Anaplasma marginale susceptible to chlortetracycline. |
| 0.5 mg/lb. daily | Beef
cattle (Over 700 lbs.): Control of active infection of anaplasmosis
caused by Anaplasma marginale susceptible to chlortetracycline. |
For calves, beef and non-lactating dairy cattle
| DOSAGE LEVEL | INDICATION FOR USE |
| 10 mg/lb. body weight daily | Treatment
of bacterial eneteritis caused by Escherichia coli and bacterial
pneumonia caused by Pasteurella multocida organisms susceptible to
chlortetracycline. (Treat for not more than 5 days. Feed approximately
400 g/ton, varying with body weight and feed consumption to provide10
mg/lb body weight per day.) |
NADA 48-480
ADM Alliance Nutrition Inc.
Quincy IL 62305-3115
NET WEIGHT 50 POUNDS (22.67Kg)
PRINCIPAL DISPLAY PANEL - 50 POUNDS
CHLORATET TM
90
Chlortetracycline
MEDICATED
TYPE A
PREMIX
NADA 48-480
ACTIVE INGREDIENTS
Chlortetracycline Calcium Complex Equivalent 90 G/lb. Chlortetracycline HCl/lb
INGREDIENTS
Dried Streptomyces Aureofaciens Fermentation Product
Calcium Carbonate, Mineral Oil.
FOR USE IN MANUFACTURE OF DRY FEED ONLY
SEE BACK OF BAG FOR DIRECTIONS FOR USE.
Manufactured by
ADM Alliance Nutrition Inc.
Quincy IL 62305-3115
NET WEIGHT 50 POUNDS (22.67Kg)
| CHLORATET 90
chlortetracycline hydrochloride granule |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NADA | NADA48-480 | 07/20/2010 | |
| Labeler - ADM Alliance Nutrition, Inc (849684428) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| ADM ALLIANCE NUTRITION, INC. | 834721284 | manufacture | |