ATUSS HS TANNATE SUSPENSION
-
hydrocodone bitartrate,
pseudoephedrine hydrochloride and
chlorpheniramine maleate suspension
Atley Pharmaceuticals, Inc.
----------
Atuss® HS Tannate Suspension Antitussive Decongestant Antihistamine Rx Only PIN 400427 ISS 10/07DESCRIPTION
Each teaspoonful (5 mL) of Atuss® HS Tannate Suspension contains:
Hydrocodone Bitartrate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 mg
Pseudoephedrine Hydrochloride . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30 mg
Chlorpheniramine Maleate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 mg
Atuss® HS Tannate Suspension is used for oral administration only.
Atuss® HS Tannate Suspension contains the following inactive ingredients: Acesulfame K, Artificial Cherry Bubblegum Flavor, Aspartame, Citric Acid, FDC Red #40, Glycerin, Hydrochloric Acid, Methylparaben, Magnesium Aluminometasilicate, Purified Water, Sodium Benzoate, Sodium Citrate Dihydrate, Sodium Hydroxide, Xanthan Gum. Plus tannic acid yielding a tannate suspension.
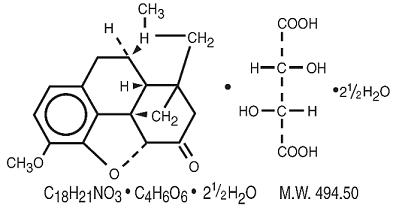
Hydrocodone Bitartrate:
4,5a–epoxy-3-methoxy-17-methylmorphinan-6-one tartrate (1:1) hydrate (2:5).
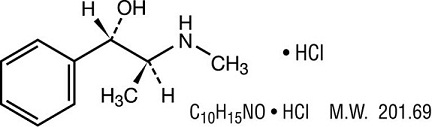
Pseudoephedrine Hydrochloride:
[S(R*, R*)]- α -[1-(methylamino)ethyl] benzenemethanol hydrochloride.
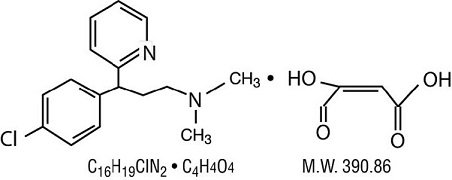
Chlorpheniramine Maleate:
2-[p-chloro- α -[2-(dimethylamino) ethyl]-benzyl] pyridine.
CLINICAL PHARMACOLOGY
Hydrocodone bitartrate is a narcotic antitussive. It acts in the medulla oblongata to elevate the cough threshold. Hydrocodone possesses narcotic analgesic properties; tolerance can develop and a potential for addiction exists. Hydrocodone is rapidly metabolized after ingestion, with trace amounts of unchanged drug in the blood and urine.
Pseudoephedrine is an α-adrenergic receptor antagonist (sympathomimetic) which produces vasoconstriction by stimulating α-receptors within the mucosa of the respiratory tract. Clinically, pseudoephedrine shrinks swollen mucous membranes, reduces tissue hyperemia, edema, and nasal congestion, and increases nasal airway patency. The vasoconstriction action of pseudoephedrine is similar to that of ephedrine. In the usual dose it has minimal vasopressor effects. Pseudoephedrine is rapidly and almost completely absorbed from the gastrointestinal tract. Acidic urine is associated with faster elimination of the drug. The drug is distributed to body tissues and fluids, including the fetal tissue, breast milk and the central nervous system (CNS).
Chlorpheniramine is an alkylamine-type antihistamine. The antihistamine in Atuss® HS Tannate Suspension acts by competing with histamine for H1 histamine receptor sites, thereby preventing the action of histamine on the cell. Clinically, chlorpheniramine suppresses the histamine-mediated symptoms of allergic rhinitis, relieving sneezing, rhinorrhea, and itching of the eyes, nose, and throat.
INDICATIONS
Atuss® HS Tannate Suspension is indicated for temporary relief of nasal congestion and cough associated with respiratory tract infections and related conditions such as sinusitis, pharyngitis, bronchitis and asthma when these conditions are complicated by tenacious mucus and/or mucous plugs and congestion. Atuss® HS Tannate Suspension is effective in a productive as well as a nonproductive cough, but is of particular value in a dry nonproductive cough which tends to injure the mucous membrane of the air passages.
CONTRAINDICATIONS
Atuss® HS Tannate Suspension is contraindicated in patients with hypersensitivity to hydrocodone, chlorpheniramine, or with hypersensitivity or idiosyncrasy to sympathomimetic amines which may be manifested by insomnia, dizziness, weakness, tremor or arrhythmias. Patients known to be hypersensitive to other opioids may exhibit cross sensitivity to Atuss® HS Tannate Suspension. Hydrocodone is contraindicated in the presence of an intracranial lesion associated with increased intracranial pressure; and whenever ventilatory function is depressed. Sympathomimetic amines are contraindicated in patients with severe hypertension, severe coronary artery disease, and patients that are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for two weeks after stopping the MAOI drug.
WARNINGS
Respiratory Depression:
At high doses or in sensitive patients, hydrocodone may produce dose-related respiratory depression by acting directly on the brain stem respiratory center. Hydrocodone also affects the center that controls respiratory rhythm, and may produce irregular and periodic breathing.
Head Injury and Increased Intracranial Pressure:
The respiratory depressant effects of narcotics and their capacity to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions or a preexisting increase in intracranial pressure. Furthermore, narcotics produce adverse reactions which may obscure the clinical course of patients with head injuries.
Acute Abdominal Conditions:
The administration of narcotics may obscure the diagnosis or clinical course of patients with acute abdominal conditions. Do not take this product for persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema, or where cough is accompanied by excessive phlegm (mucus) unless directed by a doctor. Sympathomimetic amines should be used with caution in patients with hypertension, ischemic heart disease, diabetes mellitus, increased intraocular pressure, hyperthyroidism, or prostatic hypertrophy. Sympathomimetics may produce CNS stimulation with convulsions or cardiovascular collapse with accompanying hypotension. Nervousness, dizziness or sleeplessness may occur at higher doses. Do not exceed recommended dosage.
PRECAUTIONS
Special Risk Patients:
As with any narcotic analgesic agent, Atuss® HS Tannate Suspension should be used with caution in elderly or debilitated patients, and those with severe impairment of hepatic or renal functions, hyperthyroidism, Addison’s disease, prostatic hypertrophy or urethral stricture. The usual precautions should be observed and the possibility of respiratory depression should be kept in mind. Before prescribing medication to suppress or modify cough, it is important that the underlying cause of cough is identified, that modification of cough does not increase the risk of clinical or physiologic complications, and that appropriate therapy for the primary disease is instituted. Check with physician if cough persists after medication has been used for seven days or if high fever, skin rash, or continued headache, or sore throat is present with cough. The antihistamine in Atuss® HS Tannate Suspension may exhibit additive effects with CNS depressants, including alcohol.
Phenylketonurics:
Contains Phenylalanine 25.25 mg. per 5 mL.
Information for Patients:
- Patients receiving narcotic analgesics, alcohol, tranquilizers and other CNS depressants concomitantly with Atuss® HS Tannate Suspension may exhibit CNS depression.
- Avoid alcohol while taking Atuss® HS Tannate Suspension.
- Elderly or debilitated patients and those sensitive to narcotics should take Atuss® HS Tannate Suspension with caution.
- Do not take MAOI while taking Atuss® HS Tannate Suspension.
- Keep all medications out of the reach of children. In case of accidental overdose, seek professional assistance or contact a poison control center immediately.
- Antihistamines may impair mental and physical abilities required for the performance of potentially hazardous tasks, such as driving a vehicle or operating machinery.
Drug Interactions:
- Patients receiving narcotics, antihistamines, antipsychotics, antianxiety agents or other CNS depressants (including alcohol) concomitantly with Atuss® HS Tannate Suspension may exhibit an additive CNS therapy. When combined therapy is contemplated, the dose of one or both should be reduced.
- MAOI and tricyclic antidepressants may prolong and intensify the anticholinergic (drying) effects of antihistamines.
- Beta-adrenergic blockers and MAOI may potentiate the pressor effect of pseudoephedrine.
- Concurrent use of digitalis glycosides may increase the possibility of cardiac arrhythmias.
- Sympathomimetics may reduce the hypotensive effects of guanethidine, mecamylamine, methyldopa, reserpine and veratrum alkaloids.
- Concurrent use of tricyclic antidepressants may antagonize the effects of pseudoephedrine.
- Concomitant use of antihistamines with alcohol, tricyclic antidepressants, barbiturates and other CNS depressants may have an additive effect.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
No data is available on the long-term potential of the components of Atuss® HS Tannate Suspension for carcinogenesis, mutagenesis or impairment of fertility in animals or humans.
Pregnancy: Category C:
Animal reproduction studies have not been conducted with Atuss® HS Tannate Suspension. It is also not known if Atuss® HS Tannate Suspension can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Atuss® HS Tannate Suspension should be used in pregnant woman only if the potential benefit justifies risk to the fetus.
Nursing Mothers:
Pseudoephedrine is excreted in breast milk. Use of Atuss® HS Tannate Suspension by nursing mothers is not recommended because of the higher-than-usual risk for infants from sympathomimetic amines. Since most drugs appear in breast milk and due to the possible drug dependence occurring from hydrocodone bitartrate, the physician should decide whether to discontinue nursing while administering this medication.
Pediatric Use:
Hydrocodone cough suppressants have not yet been established to be safe and effective for children under 6 years of age and are not recommended. Pseudoephedrine may be more likely to cause side effects in infants, especially newborn and premature infants, than in older children and adults.
Geriatric Use: (Ages 65 and older)
Geriatric patients may be more susceptible to the effects, especially the respiratory depressant effects and vasopressor effects of narcotic antitussives. Geriatric patients taking sympathomimetics may be more likely to experience confusion, hallucinations, seizures, and CNS depression. Geriatric patients may also be more sensitive to the effects, especially to the vasopressor effects, of sympathomimetic amines. Demonstrate safe use of a short-acting sympathomimetic formulation before use of a sustained-action formulation in elderly patients.
ADVERSE REACTIONS
The most frequent side effects observed with hydrocodone include lightheadedness, dizziness, drowsiness, sedation, nausea, and vomiting. These effects seem to be more prominent in ambulatory than in non-ambulatory patients and some of these adverse reactions may be alleviated if the patient lies down.
Pseudoephedrine may cause mild CNS stimulation, especially in those patients who are hypersensitive to sympathomimetic drugs. Nervousness, excitability, restlessness, dizziness, weakness and insomnia may also occur. Headache and drowsiness have also been reported. Large doses may cause lightheadedness, nausea and/or vomiting. Sympathomimetics have been associated with certain untoward reactions including fear, anxiety, nervousness, restlessness, tremor, weakness, pallor, respiratory difficulty, dysuria, insomnia, hallucinations, convulsions, CNS depression, arrhythmias, and cardiovascular collapse with hypotension.
Chlorpheniramine may cause slight to moderate drowsiness and is the most frequent side effect. Other possible side effects of antihistamines include:
General: Urticaria, drug rash, anaphylactic shock, photosensitivity, excessive perspiration, chills, dryness of mouth, nose and throat;
Cardiovascular: Hypotension, headache, palpitation, tachycardia, extra systoles;
Hematological: Hemolytic anemia, thrombocytopenia, agranulocytosis;
CNS: Sedation, dizziness, disturbed coordination, fatigue, confusion, restlessness, excitation, nervousness, tremor, irritability, insomnia, euphoria, paresthesia, blurred vision, diplopia, vertigo, tinnitus, hysteria, neuritis, convulsion;
Gastrointestinal: Epigastric distress, anorexia, nausea, vomiting, diarrhea, constipation;
Genitourinary: Urinary frequency, difficult urination, urinary retention, early menses;
Respiratory: Thickening of bronchial secretions, tightness of chest, wheezing and nasal stuffiness.
DRUG ABUSE AND DEPENDENCE:
Because Atuss® HS Tannate Suspension contains Hydrocodone Bitartrate, there does exist the possibility of drug dependence of the morphine type. Therefore, care should be taken to caution the patient not to increase the dosage on his own. Special care is needed when prescribing to patient with a known propensity to increase the dosage. Pseudoephedrine, like other CNS stimulants, has been abused. At high doses, subjects commonly experience an elevation of mood, a sense of increased energy and alertness, and decreased appetite. Some individuals become anxious, irritable, and loquacious. In addition to the marked euphoria, the user experiences a sense of markedly enhanced physical strength and mental capacity. With continued use, tolerance develops, the user increases the dose and toxic signs and symptoms appear. Depression may follow rapid withdrawal. Narcotic antitussives and stimulants, such as pseudoephedrine and hydrocodone, are banned and tested for by the U.S. Olympic Committee (USOC) and the National Collegiate Athletic Association (NCAA).
OVERDOSAGE:
Serious overdosage with hydrocodone is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, and sometimes bradycardia and hypotension. In severe overdosage, apnea, circulatory collapse, cardiac arrest, or death may occur. Symptoms include coma, pinpoint pupils and depressed respiration. Shock, decreased body temperature and pulmonary edema may occur.
Overdosage with pseudoephedrine may manifest itself as excessive CNS stimulation resulting in excitement, tremor, restlessness and insomnia. Other effects may include tachycardia, hypertension, pallor, mydriasis, hyperglycemia and urinary retention. Severe overdosage may cause tachypnea or hyperpnea, hallucinations, convulsions or delirium, but in some individuals there may be CNS depression with somnolence, stupor or respiratory depression. Arrhythmias (including ventricular fibrilation) may lead to hypotension and circulatory collapse. Severe hypokalemia can occur, probably due to a compartmental shift rather than a depletion of potassium. No organ damage or significant metabolic derangement is associated with pseudoephedrine overdosage.
Manifestations of antihistamine overdosage may vary from CNS depression (sedation, apnea, cardiovascular collapse) to stimulation (insomnia, hallucinations, tremors or convulsions). Other signs and symptoms may be dizziness, tinnitus, ataxia, blurred vision, and hypotension. Stimulation is particularly likely in children, as are atropine-like signs and symptoms (dry mouth; fixed, dilated pupils; flushing; hyperthermia and gastrointestinal symptoms).
Treatment:
Primary attention should be given to the reestablishment of adequate respiratory exchange through provision of a patent airway and the institution of assisted or controlled ventilation. The narcotic antagonist naloxone is a specific antidote against respiratory depression which may result from overdosage or unusual sensitivity to narcotics, including hydrocodone. Therefore, an appropriate dose of naloxone hydrochloride should be administered, preferably by the intravenous route, and simultaneously with efforts at respiratory resuscitation. Since the duration of action of hydrocodone may exceed that of the antagonist, the patient should be kept under continued surveillance and repeated doses of the antagonist should be administered as needed to maintain adequate respiration. An antagonist should not be administered in the absence of clinically significant respiratory or cardiovascular depression. Oxygen, intravenous fluids, vasopressors and other supportive measures should be employed as indicated. Gastric emptying (Syrup of Ipecac) and/or lavage is recommended as soon as possible after ingestion, even if the patient has vomited spontaneously. Either isotonic or half-isotonic saline may be used for lavage. Administration of an activated charcoal slurry is beneficial after lavage and/or emesis if less than four hours have passed since ingestion. Saline cathartics, such as Milk of Magnesia, are useful for hastening the evacuation of unreleased medication. Adrenergic receptor blocking agents are antidotes to pseudoephedrine. In practice, the most useful is the beta-blocker propanolol, which is indicated when there are signs of cardiac toxicity. In severe cases of overdosage, it is essential to monitor both the heart (by electrocardiograph) and plasma electrolytes, and to give intravenous potassium as indicated.
Vasopressors may be used to treat hypotension. Excessive CNS stimulation may be counteracted with parenteral diazepam. Stimulants should not be used. Hyperpyrexia, especially in children, may require treatment with tepid water sponge baths or a hyperthermic blanket. Apnea is treated with ventilatory support.
DOSAGE AND ADMINISTRATION:
Adults and children over age 12: 1–2 teaspoonsful every 12 hours. Children ages 6 to 12: 1/2 – 1 teaspoonful every 12 hours. Children under age 6: Not recommended.
Shake well before use.
Note: The bitartrate salt of hydrocodone, the hydrochloride salt of pseudoephedrine, and the maleate salt of chlorpheniramine are provided in a tannate suspension by means of the TCT manufacturing process. This yields a corresponding 10 mg of hydrocodone tannate, 60 mg of pseudoephedrine tannate, and 8 mg of chlorpheniramine tannate
HOW SUPPLIED:
Atuss® HS Tannate Suspension is a red, cherry bubblegum flavored suspension. Available in 16 fl. oz. (473 mL) NDC 59702-799-16 and 1/2 fl. oz. (15 mL) NDC 59702-799-15.
Storage:
Dispense in a well closed, light-resistant container. Store at controlled room temperature, 20°–25°C (68°–77°F).
WARNINGS:
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. IN CASE OF ACCIDENTAL OVERDOSE, SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
Manufactured for:
Atley Pharmaceuticals, Inc.
Ashland, VA 23005
U.S. Patent's
# 6,869,618
# 7,094,429
PIN 400427
Rx Only
ISS. 10/07
© 2006 Atley Pharmaceuticals, Inc.
REPRESENTATIVE PACKAGING
See How Supplied
section for a complete list of available packages of Atuss® HS Tannate
Suspension
NDC 59702-799-16
Atuss® HS
Tannate Suspension
Each 5mL (1
teaspoon) contains:
Hydrocodone Bitartrate 5mg
Pseudoephedrine HCI 30mg
Chlorpheniramine
Maleate 4mg
SHAKE WELL BEFORE USING
ATLEY
Rx Only
16 fl. oz. (473
mL)
USUAL DOSAGE:
Adults and children over age 12: 1-2 teaspoonsful
every 12 hours.
Children ages 6 to 12: 1/2 - 1 teaspoonful every 12 hours.
Children
under age 6: Not recommended.
See attached insert for full prescribing
information.
Pharmacist: Dispense in a well closed, light-resistant container.
WARNING:
Keep this and all medications out of the reach of children.
Store at controlled room temperature
20°-25°C (68°-77°F) (see USP)
PSL053816
400427
80310731, R.1
80310928
Lot. No.
Exp.
Mfg. for: Atley Pharmaceuticals, Inc.
Ashland, VA 23005
Utilizing: Tannate Conversion Technology

| ATUSS HS TANNATE SUSPENSION
tannate suspension suspension |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved drug other | 11/21/2006 | 08/03/2009 | |
| Labeler - Atley Pharmaceuticals, Inc. (928666536) |