CODEINE SULFATE
-
codeine sulfate tablet
Glenmark Generics Inc., USA
----------
Codeine Sulfate Tablets USPRx Only
DESCRIPTION
Each tablet contains:
Codeine Sulfate 30 or 60 mg
Chemically, Codeine Sulfate is, Morphinan-6-ol,7,8-didehydro-4,5-epozy-3-methoxy-17- methyl-,(5_,6_)-, sulfate (2:1) (salt), trihydrate, which can be represented by the following structural formula:
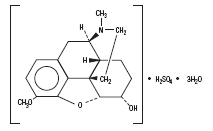
Codeine Sulfate acts as a narcotic analgesic.
Inactive Ingredients:
Croscarmellose sodium, lactose anhydrous, magnesium stearate, microcrystalline cellulose and pregelatinized starch.
CLINICAL PHARMACOLOGY
The major effects of codeine are on the central nervous system and the bowel. Opioids act as agonists, interacting with stereospecific and saturable binding sites or receptors in the brain and other tissues.
Codeine is readily absorbed from the gastrointestinal tract with the maximum analgesic effect occurring 60 minutes post administration. Codeine retains at least one half its analgesic activity when administered orally.
INDICATIONS AND USAGE
For the relief of mild to moderate pain.
CONTRAINDICATIONS
Hypersensitivity to codeine.
WARNINGS
Drug Dependence:
Codeine Sulfate can produce drug dependence of the morphine type, and therefore, has the potential for being abused. Psychic dependence, physical dependence and tolerance may develop upon repeated administration of this drug and it should be prescribed and administered with the same degree of caution appropriate to the use of other oral narcotic-containing medications. Like other narcotic-containing medications, the drug is subject to the Federal Controlled Substances Act.
Usage in ambulatory patients:
Codeine may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery. The patient using this drug should be cautioned accordingly.
Interaction with other central nervous system depressants:
Patients receiving other narcotic analgesics, general anesthetics, phenothiazines, other tranquilizers, sedative-hypnotics or other CNS depressants (including alcohol) concomitantly with codeine may exhibit an additive CNS depression. When such combined therapy is contemplated, the dose of one or both agents should be reduced.
Usage in Pregnancy:
Safe use in pregnancy has not been established relative to possible adverse effects on fetal development. Therefore codeine should not be used in pregnant women unless, in the judgement of the physician, the potential benefits outweigh the possible hazards.
Pediatric Use:
Safe dosage of this drug has not been established in children below the age of three.
PRECAUTIONS
Head injury and increased intracranial pressure:
The respiratory depressant effects of narcotics and their capacity to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions or a pre-existing increase intracranial pressure. Furthermore, narcotics produce adverse reactions which may obscure the clinical course of patients with head injuries.
Acute abdominal conditions:
The administration of codeine or other narcotics may obscure the diagnosis or clinical course in patients with acute abdominal conditions.
Special risk patients:
Codeine should be given with caution to certain patients such as the elderly or debilitated, and those with severe impairment of hepatic or renal function, hypothyroidism, Addison’s disease, and prostatic hypertrophy or urethral stricture.
ADVERSE REACTIONS
THE MAJOR HAZARDS OF CODEINE, AS OF OTHER NARCOTIC ANALGESICS, ARE RESPIRATORY DEPRESSION AND, TO A LESSOR DEGREE, CIRCULATORY DEPRESSION. RESPIRATORY ARREST, SHOCK, AND CARDIAC ARREST HAVE OCCURRED.
The most frequently observed adverse reactions include lightheadedness, dizziness, sedation, nausea, vomiting, and sweating. These effects seem to be more prominent in ambulatory patients and in those who are not suffering severe pain. In such individuals, lower doses are advisable. Some adverse reactions may be alleviated in the ambulatory patient if he lies down.
Other adverse reactions include the following:
Central Nervous System – Euphoria, dysphoria, weakness, headache, insomnia, agitation, disorientation, and visual disturbances.
Gastrointestinal – Dry mouth, anorexia, constipation, and biliary tract spasm.
Cardiovascular – Flushing of the face, bradycardia, palpitation, faintness, and syncope.
Genitourinary – Urinary retention or hesitancy, anti-diuretic effect, and reduced libido and/or potency.
Allergic – Pruritus, urticaria, other skin rashes, edema, and, rarely, hemorrhagic urticaria.
OVERDOSAGE
Signs and Symptoms: Serious overdose with codeine is characterized respiratory depression (a decrease in respiratory rate and/or tidal volume. Cheyne-Stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, and sometimes bradycardia and hypotension. In severe overdosage, apnea, circulatory collapse, cardiac arrest and death may occur.
Treatment: Primary attention should be given to the re-establishment of adequate respiratory exchange through provision of a patient airway and the institution of assisted or controlled ventilation. The narcotic antagonist naloxone is a specific antidote against respiratory depression which may result from overdosage or unusual sensitivity to narcotics, including codeine. Therefore, an appropriate dose of naloxone (usual initial adult dose: 0.4 mg) should be administered, preferably by the intravenous route and simultaneously with efforts at respiratory resuscitation. Since the duration of action of codeine may exceed that of the antagonist, the patient should be kept under continued surveillance and repeated doses of the antagonist should be administered as needed to maintain adequate respiration.
An antagonist should not be administered in the absence of clinically significant respiratory or cardiovascular depression.
Oxygen, intravenous fluids, vasopressors and other supportive measures should be employed as indicated.
Gastric emptying may be useful in removing unabsorbed drug.
DOSAGE AND ADMINISTRATION
Dosage should be adjusted accordingly to the severity of the pain and the response of the patient. It may occasionally be necessary to exceed the usual dosage recommended below in cases of more severe pain or in those patients who have become tolerant to the analgesic effect of narcotics. Codeine tablets are given orally. The usual adult dose is 15 to 60 mg every 4 hours as needed.
HOW SUPPLIED
Codeine Sulfate Tablets USP are available as white, round, beveled edge tablets.
30 mg, bottles of 100 (NDC 68462-193-01, Imprinted LV on one side and 903 on the other side)
60 mg, bottles of 100 (NDC 68462-194-01, Imprinted LV on one side and 906 on the other side)
Store at 25oC (77oF); excursions permitted to 15-30oC (59-86oF) [see USP Controlled Room Temperature].
PROTECT FROM MOISTURE
Dispense in well-closed container as defined in the USP/NF.
DEA Order Form Required
Manufactured by:
Lehigh Valley Technologies Inc.
Allentown, PA 18102
Distributed by:

Glenmark Generics Inc.
Mawhah, NJ 07430
Questions? 888-721-7115
www.glenmarkgenerics.com
April 2008
Principal Display Panel - 30 mg

Principal Display Panel - 60 mg

| CODEINE SULFATE
codeine sulfate tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Unapproved drug other | 07/01/2006 | 08/31/2011 | |
| CODEINE SULFATE
codeine sulfate tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Unapproved drug other | 07/01/2006 | 08/31/2011 | |
| Labeler - Glenmark Generics Inc., USA (835917282) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Lehigh Valley Technologies | 113290444 | ANALYSIS, MANUFACTURE | |