PHENYLEPHRINE HYDROCHLORIDE, CHLORPHERNIRAMINE MALEATE
-
phenylephrine hydrochloride and
chlorpheniramine maleate tablet
River's Edge Pharmaceuticals, LLC
----------
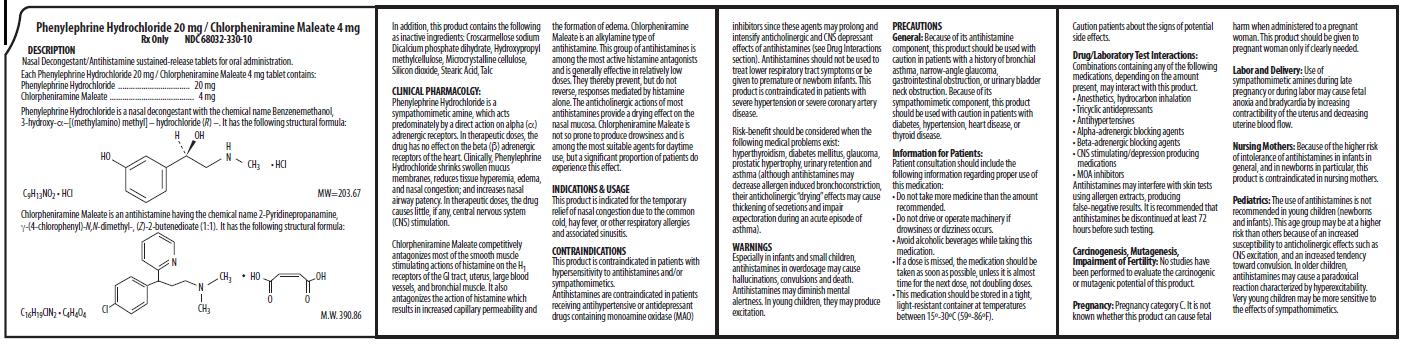
Phenylephrine Hydrochloride 20 mg /Chlorpherniramine Maleate 4mgDESCRIPTION:
Nasal Decongestant/Antihistamine sustained-release tablets for oral administration.
Each Phenylephrine Hydrochloride 20 mg / Chlorpheniramine Maleate 4 mg tablet contains:
Phenylephrine Hydrochloride .................................. 20 mg
Chlorpheniramine Maleate ........................................ 4 mg
In addition, this product contains the following
as inactive ingredients: Croscarmellose sodium
Dicalcium phosphate dihydrate, Hydroxypropyl
methylcellulose, Microcrystalline cellulose,
Silicon dioxide, Stearic Acid, Talc
CLINICAL PHARMACOLOGY:
Phenylephrine Hydrochloride is a
sympathomimetic amine, which acts
predominately by a direct action on alpha
adrenergic receptors. In therapeutic doses, the
drug has no effect on the beta adrenergic
receptors of the heart. Clinically, Phenylephrine
Hydrochloride shrinks swollen mucus
membranes, reduces tissue hyperemia, edema,
and nasal congestion; and increases nasal
airway patency. In therapeutic doses, the drug
causes little, if any, central nervous system
(CNS) stimulation.
Chlorpheniramine Maleate competitively
antagonizes most of the smooth muscle
stimulating actions of histamine on the H1
receptors of the GI tract, uterus, large blood
vessels, and bronchial muscle. It also
antagonizes the action of histamine which
results in increased capillary permeability and
the formation of edema. Chlorpheniramine
Maleate is an alkylamine type of
antihistamine. This group of antihistamines is
among the most active histamine antagonists
and is generally effective in relatively low
doses. They thereby prevent, but do not
reverse, responses mediated by histamine
alone. The anticholinergic actions of most
antihistamines provide a drying effect on the
nasal mucosa. Chlorpheniramine Maleate is
not so prone to produce drowsiness and is
among the most suitable agents for daytime
use, but a significant proportion of patients do
experience this effect.
INDICATIONS & USAGE:
This product is indicated for the temporary
relief of nasal congestion due to the common
cold, hay fever, or other respiratory allergies
and associated sinusitis.
CONTRAINDICATIONS:
This product is contraindicated in patients with
hypersensitivity to antihistamines and/or
sympathomimetics.
Antihistamines are contraindicated in patients
receiving antihypertensive or antidepressant
drugs containing monoamine oxidase (MAO)
inhibitors since these agents may prolong and
intensify anticholinergic and CNS depressant
effects of antihistamines (see Drug Interactions
section). Antihistamines should not be used to
treat lower respiratory tract symptoms or be
given to premature or newborn infants. This
product is contraindicated in patients with
severe hypertension or severe coronary artery
disease.
Risk-benefit should be considered when the
following medical problems exist:
hyperthyroidism, diabetes mellitus, glaucoma,
prostatic hypertrophy, urinary retention and
asthma (although antihistamines may
decrease allergen induced bronchoconstriction,
their anticholinergic drying effects may cause
thickening of secretions and impair
expectoration during an acute episode of
asthma).
WARNINGS:
Especially in infants and small children,
antihistamines in overdosage may cause
hallucinations, convulsions and death.
Antihistamines may diminish mental
alertness. In young children, they may produce
excitation.
PRECAUTIONS:
General: Because of its antihistamine
component, this product should be used with
caution in patients with a history of bronchial
asthma, narrow-angle glaucoma,
gastrointestinal obstruction, or urinary bladder
neck obstruction. Because of its
sympathomimetic component, this product
should be used with caution in patients with
diabetes, hypertension, heart disease, or
thyroid disease.
Information for Patients
Patient consultation should include the
following information regarding proper use of
this medication:
• Do not take more medicine than the amount
recommended.
• Do not drive or operate machinery if
drowsiness or dizziness occurs.
• Avoid alcoholic beverages while taking this
medication.
• If a dose is missed, the medication should be
taken as soon as possible, unless it is almost
time for the next dose, not doubling doses.
• This medication should be stored in a tight,
light-resistant container at temperatures
between 15º-30ºC (59º-86ºF).
Caution patients about the signs of potential
side effects.
Drug/Laboratory Test Interactions
Drug/Laboratory Test Interactions:Combinations containing any of the following
medications, depending on the amount
present, may interact with this product.
• Anesthetics, hydrocarbon inhalation
• Tricyclic antidepressants
• Antihypertensives
• Alpha-adrenergic blocking agents
• Beta-adrenergic blocking agents
• CNS stimulating/depression producing
medications
• MOA inhibitors
Antihistamines may interfere with skin tests
using allergen extracts, producing
false-negative results. It is recommended that
antihistamines be discontinued at least 72
hours before such testing.
Carcinogenesis, Mutagenesis and Impairment of Fertility
Carcinogenesis, Mutagenesis,
Impairment of Fertility: No studies have
been performed to evaluate the carcinogenic
or mutagenic potential of this product.
Pregnancy
Pregnancy: Pregnancy category C. It is not
known whether this product can cause fetal
harm when administered to a pregnant
woman. This product should be given to
pregnant woman only if clearly needed.
Labor and Delivery
Labor and Delivery: Use of
sympathomimetic amines during late
pregnancy or during labor may cause fetal
anoxia and bradycardia by increasing
contractibility of the uterus and decreasing
uterine blood flow.
Nursing Mothers
Nursing Mothers: Because of the higher risk
of intolerance of antihistamines in infants in
general, and in newborns in particular, this
product is contraindicated in nursing mothers.
Pediatrics
Pediatrics: The use of antihistamines is not
recommended in young children (newborns
and infants). This age group may be at a higher
risk than others because of an increased
susceptibility to anticholinergic effects such as
CNS excitation, and an increased tendency
toward convulsion. In older children,
antihistamines may cause a paradoxical
reaction characterized by hyperexcitability.
Very young children may be more sensitive to
the effects of sympathomimetics.
Geriatrics
Geriatrics: The elderly (60 years or older) maybe more susceptible to the vasopressor effects
of sympathomimetics. Confusion,
hallucinations, seizures and CNS depression
may be more likely to occur in geriatric patients
taking sympathomimetics. Antihistamines
may cause confusion, dizziness, sedation,
hypotension, hyperexcitability, and
anticholinergic side effects such as dryness of
the mouth and urinary retention in geriatric
patients. If these symptoms occur and
continue, or are severe, medication should
probably be discontinued.
ADVERSE REACTIONS:
The most frequent reactions include
drowsiness, lightheadedness, nausea, and
dryness of mouth. Less frequently restlessness,
nervousness, trembling, or weakness may
occur. These side effects need medical
attention only if they continue or are
bothersome.
Those indicating need for immediate medical
attention include: CNS depression (severe
drowsiness), CNS stimulation (hallucinations,
seizures), anticholinergic effects (clumsiness,
flushing of the face, shortness of breath,
troubled breathing), severe headache,
hypertension, or hypotension.
DRUG ABUSE AND DEPENDENCE:
Central nervous system stimulants such as
sympathomimetic amines have been abused.
At high doses, subjects commonly experience
an elevation of mood, a sense of increased
energy and alertness and decreased appetite.
Some individuals become anxious, irritable,
and loquacious. In addition to the marked
euphoria, the user experiences a sense of
markedly enhanced physical strength and
mental capacity. With continued use, tolerance
develops, the user increases the dose, and toxic
signs and symptoms appear. Depression may
follow rapid withdrawal.
Nasal decongestants such as Phenylephrine
Hydrochloride have been banned and tested by
the U.S. Olympic Committee (USOC) and the
National Collegiate Athletic Association
(NCAA).
OVERDOSAGE:
OVERDOSAGESigns and symptoms: This product is
comprised of pharmacologically different
products. Therefore, it is difficult to predict the
exact manifestation of symptoms in a given
individual. A description of symptoms, which
are likely to appear after ingestion of an excess
of the individual components, follows:
Overdosage with antihistamines may cause
hallucinations, convulsions or possibly death,
especially in infants and children.
Antihistamines are more likely to cause
dizziness, sedation, and hypotension in elderly
patients.
Overdosage with sympathomimetic amines
can cause cardiac arrhythmias, cerebral
hemorrhage and pulmonary edema. It can also
cause palpitations, tremor, dizziness, vomiting,
fear, labored breathing, headache, pallor,
weakness, hallucinations, and delirium.
Recommended treatment: In the event of
overdose, emergency treatment should be
started immediately. Since the action of
sustained release products may continue for as
long as 12 hours, treatment should be directed
toward reducing further absorption and
supporting the patient for at least that length
of time.
Since there is no specific antidote for overdose
with this product, treatment is symptomatic
and supportive with possible utilization of the
following:
• If the amount ingested is considered
dangerous or excessive induce vomiting with
ipecac syrup unless the patient is convulsing,
comatose, or has lost the gag reflex, in which
case perform gastric lavage.
• Gastric lavage (isotonic or 0.45% sodium
chloride solution) if patient is unable to vomit
within 3 hours of ingestion.
• Saline cathartics (milk of magnesia) are
sometimes used.
• Vasopressors to treat hypotension, however,
epinephrine should not be used since it may
further lower blood pressure.
• Oxygen and intravenous fluids
• Precaution against use of stimulants
(analeptic agents) because they may cause
seizures.
• For reflex bradycardia accompanying the
pressor response to phenylephrine atropine
may be used to block the effect.
• For excessive hypertensive effects, an
alpha-adrenergic blocker, such as
phentolamine, may be administered.
DOSAGE AND ADMINISTRATION:
Adults and adolescents 12 years of age
and older: 1 or 2 tablets every 12 hours as
directed by a physician.
Children 6 to 12 years: 1 tablet every 12
hours, as directed by a physician.
Not recommended for children under 6
years.
Tablets should not be crushed or chewed.
Do not exceed recommended doses in a
24-hour period.
HOW SUPPLIED:
Phenylephrine Hydrochloride 20 mg /
Chlorpheniramine Maleate 4 mg are supplied
as white, round shaped tablets imprinted
upper “RE 330” with lower plain w/bisect.
Bottles of 100 tablets, NDC 68032-330-10.
KEEP THIS AND ALL MEDICATION OUT OF
THE REACH OF CHILDREN.
IN CASE OF ACCIDENTAL OVERDOSE, SEEK
PROFESSIONAL ASSISTANCE OR CONTACT A
POISON CONTROL CENTER IMMEDIATELY.
Dispense in a tight, light-resistant container as
defined in the USP/NF with child resistant
closures.
Store at controlled room temperature,
15°-30°C (59°-86°F); see USP Controlled Room
Temperature. Avoid exposure to heat.
Manufactured for:
Rivers Edge Pharmaceuticals, LLC
Suwanee, GA 30024
PI 284 Rev. 06/08
330-11
Label:
NDC 68032-330-10 100 Tablets
PHENYLEPHRINE HCl 20 mg/CHLORPHENIRAMINE MALEATE 4 mg
TABLETS
Rx ONLY
Nasal Decongestant/Antihistamine
Each Extended-Release Tablet Contains:
Phenylephrine Hydrochloride ........... 20 mg
Chlorpheniramine Maleate ................. 4 mg
68032 330-10
DOSAGE AND AMINISTRATION
Adults and adolescents 12 years of age and older: 1 or 2 tablets
every 12 hours as directed by a physician.
Children 6 to 12 years: 1 tablet every 12 hours, as directed by a physician.
Not recommended for children under 6 years.
See product literature for full prescribing information.
KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN.
IN CASE OF ACCIDENTAL OVERDOSE, SEEK PROFESSIONAL ASSISTANCE OR CONTACT
A POISON CONTROL CENTER IMMEDIATELY.
Dispense in a tight, light-resistant container as defined in the USP/NF with child resistant
closures.
Store at controlled room temperature, 15°-30°C (59°-86°F); see USP Controlled Room
Temperature. Avoid exposure to heat.
Manufactured for:
Rivers Edge Pharmaceuticals, LLC
Suwanee, GA 30024
PL 284 Rev. 06/08

Inserts 1 & 2:
Insert1 text here:
| PHENYLEPHRINE HYDROCHLORIDE, CHLORPHERNIRAMINE MALEATE
phenylephrine hydrochloride 20mg, chlorpherniramine maleate 4mg tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved other | 09/03/2008 | ||
| Labeler - River's Edge Pharmaceuticals, LLC (133879135) |