LAVOCLEN 4
-
benzoyl peroxide cream
LAVOCLEN 8
-
benzoyl peroxide cream
LAVOCLEN 4 ACNE WASH KIT
-
benzoyl peroxide cream
LAVOCLEN 8 ACNE WASH KIT
-
benzoyl peroxide cream
Prasco Laboratories
----------
Lavoclen-4 Creamy Wash (benzoyl peroxide 4%) Lavoclen-8 Creamy Wash (benzoyl peroxide 8%)ACNE WASH FOR TOPICAL USE
Rx Only
DESCRIPTION
Lavoclen-4 Creamy Wash and Lavoclen-8 Creamy Wash are topical preparations containing benzoyl peroxide as the active ingredient. Lavoclen-4 Creamy Wash and Lavoclen-8 Creamy Wash contain: 4% and 8% benzoyl peroxide, respectively, in a lathering cream vehicle containing cetearyl alcohol, cocamidopropyl betaine, corn starch, dimethyl isosorbide, glycerin, glycolic acid, hydrogenated castor oil, imidurea, lactic acid, methylparaben, mineral oil, PEG-14M, potassium lauryl sulfate, purified water, sodium hydroxide, sodium lauryl sulfate, sodium PCA, titanium dioxide.
The structural formula of benzoyl peroxide is:
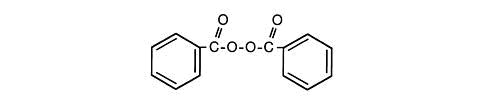
CLINICAL PHARMACOLOGY
The exact method of action of benzoyl peroxide in acne vulgaris is not known. Benzoyl peroxide is an antibacterial agent with demonstrated activity against Propionibacterium acnes. This action, combined with the mild keratolytic effect of benzoyl peroxide is believed to be responsible for its usefulness in acne.
Benzoyl peroxide is absorbed by the skin where it is metabolized to benzoic acid and excreted as benzoate in the urine.
INDICATIONS AND USAGE
Lavoclen-4 Creamy Wash and Lavoclen-8 Creamy Wash are indicated for use in the topical treatment of mild to moderate acne vulgaris. Lavoclen-4 Creamy Wash or Lavoclen-8 Creamy Wash may be used as an adjunct in acne treatment regimens including antibiotics, retinoic acid products, and sulfur/salicylic acid containing preparations.
CONTRAINDICATIONS
Lavoclen-4 Creamy Wash and Lavoclen-8 Creamy Wash should not be used in patients who have shown hypersensitivity to benzoyl peroxide or to any of the other ingredients in the product.
PRECAUTIONS
General - For external use only. Avoid contact with eyes and mucous membranes. AVOID CONTACT WITH HAIR, FABRICS OR CARPETING AS BENZOYL PEROXIDE WILL CAUSE BLEACHING.
Carcinogenesis, Mutagenesis, Impairment of Fertility - Based upon all available evidence, benzoyl peroxide is not considered to be a carcinogen. However, data from a study using mice known to be highly susceptible to cancer suggest that benzoyl peroxide acts as a tumor promoter. The clinical significance of the findings is not known.
Pregnancy: Category C - Animal reproduction studies have not been conducted with benzoyl peroxide. It is also not known whether benzoyl peroxide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Benzoyl peroxide should be used by a pregnant woman only if clearly needed.
Nursing Mothers - It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when benzoyl peroxide is administered to a nursing woman.
Pediatric Use - Safety and effectiveness in children below the age of 12 have not been established.
ADVERSE REACTIONS
Contact sensitization reactions are associated with the use of topical benzoyl peroxide products and may be expected to occur in 10 to 25 of 1000 patients. The most frequent adverse reactions associated with benzoyl peroxide use are excessive erythema and peeling which may be expected to occur in 5 of 100 patients. Excessive erythema and peeling most frequently appear during the initial phase of drug use and may normally be controlled by reducing frequency of use.
DOSAGE AND ADMINISTRATION
Shake well before using. Wash the affected areas once a day during the first week, and twice a day therafter as tolerated. Wet skin areas to be treated; apply Lavoclen-4 Creamy Wash or Lavoclen-8 Creamy Wash, work to a full lather, rinse thoroughly and pat dry. Frequency of use should be adjusted to obtain the desired clinical response. Clinically visible improvement will normally occur by the third week of therapy. Maximum lesion reduction may be expected after approximately eight to twelve weeks of drug use. Continuing use of the drug is normally required to maintain a satisfactory clinical response.
HOW SUPPLIED
Lavoclen-4 Creamy Wash is supplied as:
- 170.1 g (6.0 oz) tubes NDC 66993-926-06
- Acne Wash Kit, containing: NDC 66993-928-98
Lavoclen-4 Creamy Wash 170.1 g (6.0 oz) tube and
Soap Free Cleanser Lotion 106.6 mL (3.6 Fl Oz)
Lavoclen-8 Creamy Wash is supplied as:
- 170.1 g (6.0 oz) tubes NDC 66993-927-06
- Acne Wash Kit, containing: NDC 66993-929-98
Lavoclen-8 Creamy Wash 170.1 g (6.0 oz) tube and
Soap Free Cleanser Lotion 106.6 mL (3.6 Fl Oz)
Store at controlled room temperature, 15°-30°C (59°-86°F).
U.S. Patent No. 6,433,024
Manufactured for: Prasco Laboratories, Mason, OH 45040 USA
Manufactured by: Stiefel Laboratorires, Inc., Coral Gables, FL 33134
Iss. 01/09
302660
Principal Display Panel
NDC 66993-926-06
LavoclenTM-4
Creamy Wash
(benzoyl peroxide 4%)
ACNE WASH FOR TOPICAL USE
Rx only
Net Wt. 170.1 g (6.0 oz)
Usual Dosage: Wash affected areas once or twice daily or as directed by physician. (See Package Insert)
Contains: Benzoyl Peroxide 4% in a cream vehicle containing Cetearyl Alcohol, Cocamidopropyl Betaine, Corn Starch, Dimethyl Isosorbide, Glycerin, Glycolic Acid, Hydrogenated Castor Oil, Imidurea, Lactic Acid, Methylparaben, Mineral Oil, PEG-14M, Potassium Lauryl Sulfate, Purified Water, Sodium Hydroxide, Sodium Lauryl Sulfate, Sodium PCA, Titanium Dioxide.
Caution: Avoid contact with eyes and mucous membranes. AVOID CONTACT WITH HAIR, FABRICS OR CARPETING AS BENZOYL PEROXIDE WILL CAUSE BLEACHING.
Store at controlled room temperature, 15o -30oC (59o – 86oF).
Expiration date and lot number on crimp.
Mfd. for: Prasco Laboratories, Mason, OH 45040 USA
Mfd. by: Stiefel Laboratories, Inc., Coral Gables, FL 33134
U.S. Patent No. 6,433,024
Iss. 01/09 302659

Principal Display Panel
NDC 66993-928-98
LavoclenTM-4
(benzoyl peroxide 4%)
Acne Wash Kit
FOR TOPICAL USE
Rx only
One Unit
NEW FORMULATION
Convenience Kit includes:
LavoclenTM-4
Creamy Wash
(benzoyl peroxide 4%)
ACNE WASH FOR TOPICAL USE
Rx Only
Net Wt. 170.1 g (6.0 oz)
Soap-Free
Cleanser Lotion
Gentle Soap-Free Cleanser
Do Not Break Up
Soap-Free Cleanser Lotion
Gentle Soap-Free Cleanser
DIRECTIONS: Shake well. Apply a liberal amount to the skin and rub gently. Remove excess lotion with a soft cloth or tissue. When using with water, apply Soap-Free Cleanser Lotion to the skin, then moisten with warm water and gently rub to form a lather. Rinse with warm water and pat dry with a soft cloth.
INGREDIENTS: Water, PEG-75, stearyl alcohol, sodium cocoyl isethionate, methylparaben, propylparaben, butylparaben.
LavoclenTM-4
Creamy Wash
(benzoyl peroxide 4%)
ACNE WASH FOR TOPICAL USE
Usual Dosage: Wash affected areas once or twice daily or as directed by physician. (See Package Insert.)
Contains: Benzoyl Peroxide 4% in a cream vehicle containing Cetearyl Alcohol, Cocamidopropyl Betaine, Corn Starch, Dimethyl Isosorbide, Glycerin, Glycolic Acid, Hydrogenated Castor Oil, Imidurea, Lactic Acid, Methylparaben, Mineral Oil, PEG-14M, Potassium Lauryl Sulfate, Purified Water, Sodium Hydroxide, Sodium Lauryl Sulfate, Sodium PCA, Titanium Dioxide.
Caution: Avoid contact with eyes and mucous membranes. AVOID CONTACT WITH HAIR, FABRICS OR CARPETING AS BENZOYL PEROXIDE WILL CAUSE BLEACHING.
Store at controlled room temperature, 15o -30oC (59o – 86oF).
Expiration date and lot number on crimp.
Mfd. for: Prasco Laboratories, Mason, OH 45040 USA
Mfd. by: Stiefel Laboratories, Inc., Coral Gables, FL 33134
U.S. Patent No. 6,433,024
One Unit – Do Not Break Up
Mfd. for: Prasco Laboratories
Mason, OH 45040 USA
Mfd. by: Stiefel Laboratories, Inc.
Coral Gables, FL 33134
Iss. 01/09

Soap-Free Cleanser Lotion
Gentle Soap-Free Cleanser
106.6 mL (3.6 fl oz)
DIRECTIONS: Shake well. Apply a liberal amount to the skin and rub gently. Remove excess lotion with a soft cloth or tissue. When using with water, apply Soap-Free Cleanser Lotion to the skin, then moisten with warm water and gently rub to form a lather. Rinse with warm water and pat dry with a soft cloth.
INGREDIENTS: Water, PEG-75, stearyl alcohol, sodium cocoyl isethionate, methylparaben, propylparaben, butylparaben.
Mfd. for: Prasco Laboratories
Mason, OH 45040 USA
Mfd. by: Stiefel Laboratories, Inc.
Coral Gables, FL 33134
Iss. 01/09

| LAVOCLEN
4
benzoyl peroxide cream |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Unapproved drug other | 07/01/2009 | ||
| LAVOCLEN
8
benzoyl peroxide cream |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Unapproved drug other | 07/01/2009 | ||
| LAVOCLEN
4 ACNE WASH KIT
benzoyl peroxide cream |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Unapproved drug other | 07/01/2009 | ||
| LAVOCLEN
8 ACNE WASH KIT
benzoyl peroxide cream |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Unapproved drug other | 07/01/2009 | ||
| Labeler - Prasco Laboratories (065969375) |