JAY-PHYL
-
dyphylline and
guaifenesin syrup
JayMac Pharmaceuticals LLC
----------
Jay-Phyl SyrupJay-Phyl Syrup
Bronchodilator/ExpectorantRx Only
DESCRIPTION:
Jay-Phyl Syrup is a bronchodilator/expectorant combination supplied as a Orange, Vanilla flavored,
alcohol free and gluten free liquid for oral administration.
Each teaspoonful (5 mL) contains:
Dyphylline........... 100 mg
Guaifenesin........... 50 mg
Inactive ingredients: Glycerin, Propylene Glycol, Sorbitol, Citric Acid, Sodium Citrate, Sodium
Saccharin, Vanilla Flavor, FD and C Yellow No. 6, Purified Water.
Dyphylline is 7-(2,3-dihydroxypropyl)-theophylline, a white, extremely bitter, amorphous powder that is fully
soluble in water and soluble in alcohol to the extent of 2 g/100 mL. Dyphylline forms a neutral solution that
is stable in gastrointestinal fluids over a wide range of pH. It has the following chemical structure:
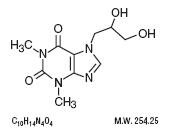
Guaifenesin is an expectorant which occurs as a white to slightly gray, crystalline powder, having a bitter
taste. It may have a slight characteristic odor. It is soluble in water, alcohol, chloroform, glycerin, and
propylene glycol. The chemical name is 1,2-Propanediol, 3-(2-methoxyphenoxy)-, (±)-. It has the following
chemical structure:
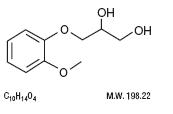
CLINICAL PHARMACOLOGY
Dyphylline is a xanthine derivative with pharmacological actions similar to theophylline and othermembers of this class of drugs. Its primary action is that of bronchodilation, but it also exhibits
peripheral vasodilatory and other smooth muscle relaxant activity to a lesser degree. The
bronchodilatory action of dyphylline, as with other xanthines, is thought to be mediated through
competitive inhibition of phosphodiesterase with a resulting increase in cyclic AMP producing
relaxation of bronchial smooth muscle.
Dyphylline was reported to be the least toxic of seven theophylline derivatives, including the piperazine,
N, N-diethylamino ethyl and the 2-hydroxy ethyl derivatives. The toxicity of dyphylline is only one-fifth
that of aminophylline as determined intraperitoneally in mice, and only one-half as toxic in rats. Unlike
the hydrolysable salts of theophylline, dyphylline is not converted to free theophylline in vivo. It is
absorbed rapidly in therapeutically active form and in healthy volunteers reaches a mean peak plasma
concentraion of 17.1 mcg/mL in approximately 45 minutes following a single oral dose of 1000 mg of
dyphylline.
Dyphylline exerts its bronchodilatory effects directly and, unlike theophylline, is excreted unchanged
by the kidneys without being metabolized by the liver. Because of this, dyphylline pharmacokinetics
and plasma levels are not influenced by various factors that affect liver function and hepatic enzyme
activity, such as smoking, age, or concomitant use of drugs which affect liver function.
The elimination half-life of dyphylline is approximately two hours (1.8-2.1 hr) and approximately 88%
of a single oral dose can be recovered from the urine unchanged. The renal clearance would be
correspondingly reduced in patients with impaired function. In anuric patients, the half-life may be
increased 3 to 4 times normal. Dyphylline plasma levels are dose-related and generally predictable.
The therapeutic range of plasma levels within which dyphylline can be expected to produce effective
bronchodilation has not been determined.
Dyphylline plasma concentrations can be accurately determined using high pressure liquid
chromatography (HPLC) or gas-liquid chromatography (GLC). Guaifenesin is an expectorant whose
action helps increase the output of thin respiratory tract fluid to facilitate mucociliary clearance and
removal of inspissated mucus.
Guaifenesin is an expectorant which increases respiratory tract fluid secretions and helps to loosen
phlegm and bronchial secretions. By reducing the viscosity of secretions, guaifenesin increases the
efficiency of the cough reflex and of ciliary action in removing accumulated secretions from the trachea
and bronchi. Guaifenesin is readily absorbed from the gastrointestinal tract and is rapidly metabolized
and excreted in the urine. Guaifenesin has a plasma half-life of one hour. The major urinary metabolite
is p-(2-methoxyphenoxy) lactic acid.
INDICATIONS AND USAGE:
Jay-Phyl Syrup is indicated as a bronchodilator-expectorant for treating bronchial asthma,emphysema, bronchitis, pneumonitis and other related bronchopulmonary insufficiency conditions.
Jay-Phyl Syrup acts to dilate bronchioles and liquefy mucus, giving relief from dyspnea,
non-productive cough and tracheobronchial irritation.
CONTRAINDICATIONS:
As with other theophylline-type drugs, combining dyphylline with epehdrine or other sympathomimeticdrugs can cause excessive CNS stimulation. Such combinations are contraindicated in children unless
accompanied by sufficient sedation to prevent undue CNS stimulation.
WARNINGS
This product is not indicated in the management of status asthmaticus, which is a seriousmedical emergency.
Although the relationship between plasma levels of dyphylline and appearance of toxicity is
unknown, excessive doses may be expected to be associated with an increased risk of
adverse effects.
PRECAUTIONS:
General: Use this product with caution in patients with severe cardiac disease, hypertension,glaucoma, hypothyroidism, severe renal and hepatic disease, acute myocardial injury or peptic
ulcer. Do not use in children under age six. Do not exceed 3 mg of dyphylline per pound
of body weight daily in older children. Because the xanthines also act as diuretics, special
precaution regarding hydration and avoidance of acidosis should be observed in children. The
long term use of xanthine derivatives may result in a cumulative effect with increase in adverse
reactions, as well as the development of tolerance.
Drug Interactions:
Synergism between xanthine bronchodilators (e.g., theophylline), ephedrine and other sympathomimeticbronchodilators has been reported. This should be considered whenever these agents are prescribed
concomitantly. Concurrent administration of dyphylline and probenecid, which competes for tubular secretion,
has been shown to increase plasma half-life and dyphylline (See Clinical Pharmacology).
Carcinogenesis, Mutagenesis, Impairment of Fertility:
No long-term animal studies have been performed with this product.Pregnancy: Teratogenic effects- Pregnancy Category C.
Animal reproduction studies have not been conducted with this formulation. It is also not known whetherthis product can cause fetal harm when administered to a pregnant woman or can affect reproduction
capacity. This medication should be given to a pregnant woman only if clearly needed.
Nursing Mothers:
Dyphylline is present in human milk at approximately twice the maternal plasma concentration.Caution should be exercised when this product is administered to a nursing woman.
Pediatric Use:
Safety and effectiveness in children below the age of six have not been established.Use caution when administering to children six years of age or older.
ADVERSE REACTIONS
This formulation may cause nausea, headache, cardiac palpitation and CNS stimulation.Postprandial administration may help avoid gastric discomfort.
The following adverse reactions which have been reported with other xanthine bronchodilators,
and which have most often been related to excessive drug plasma levels, should be considered
as potential adverse effects when dyphylline is administered.
Gastrointestinal: nausea, vomiting, epigastric pain, hematemesis, diarrhea.
Central Nervous System: headache, irritability, restlessness, insomnia, hyperexcitability,
agitation, muscle twitching, generalized clonic and tonic convulsions.
Cardiovascular: palpitation, tachycardia, extrasystoles, flushing, hypotension, circulatory failure,
ventricular arrhythmias.
Respiratory: tachypnea.
Renal: albuminuria, gross and microscopic hematuria, diuresis.
Other: hyperglycemia, inappropriate ADH syndrome.
Large doses of Guaifenesin may produce emesis, but gastrointestinal upset at ordinary dosage
levels is rare.
OVERDOSAGE
Signs and Symptoms: Restlessness, anorexia, nausea, vomiting, diarrhea, insomnia, irritability,and headache are typical symptoms resulting from a xanthine drug overdose. Marked overdosage
with resulting severe toxicity has produced agitation, severe vomiting, dehydration, excessive thirst,
tinnitus, cardiac arrhythmias, hyperthermia, diaphoresis, and generalized clonic and tonic
convulsions. Cardiovascular collapse has also occurred, with some fatalities. Seizures have
occured in some cases associated with very high theophylline plasma concentrations, without
any premonitory symptoms of toxicity.
Treatment: There is no specific antidote for overdosage with drugs of the xanthine class.
Symptomatic treatment and general supportive measures should be instituted with careful
monitoring and maintenance of vital signs, fluids and electrolytes. The stomach should be
emptied by inducing emesis if the patient is conscious and responsive, or by gastric lavage,
taking care to protect against aspiration, especially in stuporous or comatose patients.
Maintenance of an adequate airway is essential in case oxygen or assisted respiration is needed.
Sympathomimetic agents should be avoided but sedatives such as short acting barbiturates may
be useful.
Dypyhlline is dialyzable and although not recommended as routine procedure in overdosage cases,
hemodialysis may be of some benefit with severe intoxication is present or when the patient has
not responded to general supportive and symptomatic treatment.
DOSAGE AND ADMINISTRATION:
The usual adult dose is 1 or 2 teaspoonfuls of liquid 3 or 4 times a day. In severe cases, dosage maybe doubled or tripled if necessary. Maintenance dosage should be adjusted according to patient response.
Use in Children:
Altough pediatric dosages of dyphylline are established, no firm dosage for a combination of dyphylline
and guaifenesin USP can be recommended for children under the age of six. Dosage for children over
six may be calculated on the basis of 2 to 3 mg of dyphylline per pound of body weight daily in divided doses.
HOW SUPPLIED:
Jay-Phyl Syrup is supplied as a orange, vanilla flavored, alcohol free and gluten free liquid in pintbottles, NDC 64661-0814-16 and in 15 mL bottles, NDC 64661-0814-15.
Rx Only
WARNING: KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
IN CASE OF ACCIDENTAL OVERDOSE, SEEK PROFESSIONAL ASSISTANCE OR
CONTACT A POISON CONTROL CENTER IMMEDIATELY.
STORAGE:
Store at controlled room temperature 15o-30oC (59o-86oF).Tamper evident by foil seal under cap. Do not use if foil seal is broken or missing.
Manufactured by:
Great Southern Laboratories
Houston, TX 77099
Manufactured for:
JayMac Pharmaceuticals, Inc.
Sunset, LA 70584
Rev. 10/08
PRODUCT PACKAGING:
The packaging below represents the labeling currently used:Principal Display Panel and Side Panel for 15 mL Label:
NDC 64661-814-15
Jay-Phyl Syrup
Each 5 mL contains:
Dyphylline............. 100 mg
Guaifenesin........... 50 mg
Sugar and Alcohol Free
Rx Only
1/2 fl oz (15 mL)
DOSAGE AND ADMINISTRATION:
Adults: 2 teaspoonfuls (10 mL) 3 or 4 times daily. Children 6 to 12 should be started on low doses
which are gradually increased to the lowest effective dose, not to exceed 10mg/kg/day in divided
doses. Not recommended for pediatric patients under 6 years.
See package insert for full prescribing information.
Store at controlled room temperature, 15o-30oC (59o-86oF).
Tamper evident by foil seal under cap. Do not use if foil seal is broken or missing.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
IN CASE OF ACCIDENTAL OVERDOSE, CONTACT A POISON CONTROL
CENTER AND SEEK PROFESSIONAL ASSISTANCE IMMEDIATELY.
Manufactured for:
JayMac Pharmaceuticals
Sunset, LA 70584 Rev. 11/07
PROFESSIONAL SAMPLE
NOT FOR RESALE

| JAY-PHYL
dyphylline and guaifenesin syrup |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved drug other | 05/02/2006 | ||
| Labeler - JayMac Pharmaceuticals LLC (830767260) |
| Registrant - Great Southern Laboratories (056139553) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Great Southern Laboratories | 056139553 | manufacture | |