PERRIGO SODIUM SULFACETAMIDE AND SULFUR
-
sodium sulfacetamide and sulfur
Perrigo New York Inc
----------
SODIUM SULFACETAMIDE 10% AND SULFUR 5% LOTIONSodium Sulfacetamide 10% and Sulfur 5% Lotion
Rx Only
DESCRIPTION
Each mL of Sodium Sulfacetamide 10% and Sulfur 5% Lotion as dispensed contains 100 mg of sodium sulfacetamide and 50 mg of sulfur in a tinted lotion of 2-bromo-2-nitropropane-1,3-diol, butylparaben, colloidal activated attapulgite, diethanolamine, hydroxyethyl cellulose, iron oxides, lauramide DEA, methylparaben, polyethylene glycol 400 monolaurate, propylene glycol, purified water, simethicone emulsion, sodium chloride, sodium metabisulfite, sodium polynaphthalenesulfonate, talc, titanium dioxide, xanthan gum, and zinc oxide. Color Tinter contains an additional inactive ingredient, polyethylene glycol 400.
Sodium sulfacetamide is a sulfonamide with antibacterial activity while sulfur acts as a keratolytic agent. Chemically sodium sulfacetamide is N’-[(4-aminophenyl) sulfonyl]-acetamide, monosodium salt, monohydrate.
The structural formula is: 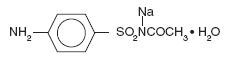
CLINICAL PHARMACOLOGY
The most widely accepted mechanism of action of sulfonamides is the Woods-Fildes theory which is based on the fact that sulfonamides act as competitive antagonists to para-aminobenzoic acid (PABA), an essential component for bacterial growth. While absorption through intact skin has not been determined, sodium sulfacetamide is readily absorbed from the gastrointestinal tract when taken orally and excreted in the urine, largely unchanged. The biological half-life has variously been reported as 7 to 12.8 hours.
The exact mode of action of sulfur in the treatment of acne is unknown, but it has been reported that it inhibits the growth of Propionibacterium acnes and the formation of free fatty acids.
INDICATIONS AND USAGE
Sodium Sulfacetamide 10% and Sulfur 5% Lotion is indicated in the topical control of acne vulgaris, acne rosacea and seborrheic dermatitis.
CONTRAINDICATIONS
Sodium Sulfacetamide 10% and Sulfur 5% Lotion is contraindicated for use by patients having known hypersensitivity to sulfonamides, sulfur, or any other component of this preparation. This drug is not to be used by patients with kidney disease.
WARNINGS
Although rare, sensitivity to sodium sulfacetamide may occur. Therefore, caution and careful supervision should be observed when prescribing this drug for patients who may be prone to hypersensitivity to topical sulfonamides. Systemic toxic reactions such as agranulocytosis, acute hemolytic anemia, purpura hemorrhagica, drug fever, jaundice, and contact dermatitis indicate hypersensitivity to sulfonamides. Particular caution should be employed if areas of denuded or abraded skin are involved.
FOR EXTERNAL USE ONLY. Keep away from eyes. Keep out of reach of children.
Keep bottle tightly closed.
Contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
PRECAUTIONS
General
If irritation develops, use of the product should be discontinued and appropriate therapy instituted. Patients should be carefully observed for possible local irritation or sensitization during long-term therapy. The object of this therapy is to achieve desquamation without irritation, but sodium sulfacetamide and sulfur can cause reddening and scaling of epidermis. These side effects are not unusual in the treatment of acne vulgaris, but patients should be cautioned about the possibility.
Carcinogenesis, Mutagenesis and Impairment of Fertility
Long-term studies in animals have not been performed to evaluate carcinogenic potential.
Pregnancy
Teratogenic effects
Pregnancy Category C
Animal reproduction studies have not been conducted with Sodium Sulfacetamide 10% and Sulfur 5% Lotion. It is also not known whether this drug can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. This drug should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether sodium sulfacetamide is excreted in human milk following topical use of Sodium Sulfacetamide 10% and Sulfur 5% Lotion. However, small amounts of orally administered sulfonamides have been reported to be eliminated in human milk. In view of this and because many drugs are excreted in human milk, caution should be exercised when this drug is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients under the age of 12 have not been established.
ADVERSE REACTIONS
Although rare, sodium sulfacetamide may cause local irritation.
DOSAGE AND ADMINISTRATION
Shake well before using. Apply a thin film to affected areas with light massaging to blend in each application 1 to 3 times daily, or as directed by a physician. Each package contains a color tinter which enables the patient to alter the basic shade of the lotion so that it matches the skin color exactly.
(Important to the Pharmacist: At the time of dispensing, add contents of sodium sulfacetamide vial* to the bottle. Shake well and/or stir with a glass rod to insure uniform dispersion. Place expiration date of four (4) months on bottle label.)
*Sodium Sulfacetamide vial contains 2.1 g of sodium sulfacetamide.
HOW SUPPLIED
Sodium Sulfacetamide 10% and Sulfur 5% Lotion is available
as follows: 25 g bottle (NDC 45802-978-01)
Store at 20°-25°C (68°-77°F) [see USP Controlled Room Temperature].
MANUFACTURED BY
STIEFEL LABORATORIES, INC.,
CORAL GABLES, FL 33134
DISTRIBUTED BY
PERRIGO®
ALLEGAN, MI 49010
Principal Display Panel - 25 g Carton
Sodium Sulfacetamide 10% and Sulfur 5% Lotion
Description: VIal contains sodium sulfacetamide. Prior to dispensing, add contents of vial to tlotion. The completed formulation then contains sodium sulfacetamide 10% and precipitated sulfur 5%.
Net Wt. 25 g (as dispensed)
Rx Only
Sodium Sulfacetamide 10% and Sulfur 5% Loation - 25 g Carton
Principal Display Panel - 25 g Label
Sodium Sulfacetamide 10% and Sulfur 5% Lotion
Net Wt. 25 g (as dispensed)
Rx Only

Sodium Sulfacetamide 10% and Sulfur 5% Loation - 25 g Label
Principal Display Panel - 2.1 g Vial
Sodium Sulfacetamide
Rx Only

Sodium Sulfacetaminde - 2.1 g Vial
Principal Display Panel - 2.5 g Color Tinter Tube
Color Tinter
(Supply separately to patient)
Net Wt. 2.5 g
Manufactured by Stiefel Lab, Inc.
Coral Gables, FL 33134
Distributed by Perrigo®
Allegan, MI 49010
Sodium Sulfacetamide - 2.5 g Color Tinter Tube
| PERRIGO SODIUM SULFACETAMIDE AND SULFUR
sodium sulfacetamide and sulfur kit |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Unapproved drug other | 10/08/2008 | ||
| Labeler - Perrigo New York Inc (078846912) |