SODIUM BICARBONATE
-
sodium bicarbonate injection
Amphastar Pharmaceuticals, Inc.
----------
Sodium Bicarbonate Injection, USP
[84 mg NaHCO3 = 1 mEq HCO3-]
FOR INTRAVENOUS USE
DESCRIPTION
Sodium Bicarbonate Injection, USP, is a sterile, aqueous, pyrogen-free preparation of sodium bicarbonate (NaHCO3). It is available in three hypertonic solutions with concentrations expressed as follows:
| 4.2% | 0.5 | mEq (42 mg) / mL | 1000 mOsmol /L (calc.) |
| 5 | mEq (0.42 g) / 10 mL | ||
| 8.4% | 1 | mEq (84 mg) / mL | 2000 mOsmol /L (calc.) |
| 10 | mEq (0.84 g) / 10 mL | ||
| 50 | mEq (4.2 g) / 50 mL |
The pH of the above solutions may have been adjusted by means of added carbon dioxide and is approximately 7.8 (USP pH limits: between 7.0 and 8.5).
These preparations contain no preservatives and are intended only as single dose vials; once the units are assembled and used, any remaining portion of the solution must be discarded with the integral units.
CLINICAL PHARMACOLOGY
Sodium bicarbonate therapy increases plasma bicarbonate, buffers excess hydrogen ion concentration, raises blood pH and reverses the clinical manifestations of metabolic acidosis. It is also useful in patients who require alkalinization of body fluids for other reasons.
Pharmacokinetics
Sodium bicarbonate in water dissociates to provide sodium (Na+) and bicarbonate (HC03-) ions. Sodium is the principal cation of the extracellular fluid. Bicarbonate is a normal constituent of body fluids and the normal plasma level ranges from 24 to 31 mEq/L. Plasma concentration is regulated by the kidney. Bicarbonate anion, at a proper concentration of hydrogen ion (H+), may be converted to carbonic acid (H2CO3), then to its volatile form, carbon dioxide (CO2) excreted by the lung. Normally, a ratio of 1:20 (carbonic acid:bicarbonate) is present in the extracellular fluid. In a healthy adult with normal kidney function, practically all the glomerular filtered bicarbonate ion is reabsorbed; less than 1% is excreted in the urine.
INDICATIONS AND USAGE
Sodium Bicarbonate Injection, USP, is indicated in the treatment of metabolic acidosis which may occur in severe renal disease, uncontrolled diabetes, circulatory insufficiency due to shock or severe dehydration, extracorporeal circulation of blood, cardiac arrest and severe primary lactic acidosis. Sodium bicarbonate is further indicated in the treatment of certain drug intoxications, including barbiturates (where dissociation of the barbiturate-protein complex is desired), in poisoning by salicylates or methyl alcohol, and in hemolytic reactions requiring alkalinization of the urine to diminish nephrotoxicity of blood pigments. Urinary alkalinization is also used in methotrexate therapy to prevent nephrotoxicity. Sodium bicarbonate also is indicated in severe diarrhea, which is often accompanied by a significant loss of bicarbonate.
Treatment of metabolic acidosis should, if possible, be superimposed on measures designed to control the basic cause of the acidosis - e.g., insulin in uncomplicated diabetes, blood volume restoration in shock. But since an appreciable time interval may elapse before all of the ancillary effects are brought about, bicarbonate therapy is indicated to minimize risks inherent to the acidosis itself.
Vigorous bicarbonate therapy is required in any form of metabolic acidosis where a rapid increase in plasma total CO2 content is crucial - e.g., cardiac arrest, circulatory insufficiency due to shock or severe dehydration, and in severe primary lactic acidosis or severe diabetic acidosis.
CONTRAINDICATIONS
Sodium bicarbonate is contraindicated in patients: losing chloride by vomiting or from continuous gastrointestinal suction; receiving diuretics known to produce a hypochloremic alkalosis; with metabolic and respiratory alkalosis; with hypocalcemia in which alkalosis may produce tetany.
WARNINGS
In neonates and children under two years of age, rapid injection (10 mL/min) of hypertonic sodium bicarbonate solutions may produce hypernatremia, a decrease in cerebrospinal fluid pressure and possible intracranial hemorrhage. The rate of administration in such patients should therefore be limited to no more than 8 mEq / kg / day. A 4.2% solution may be preferred for such slow administration.
Cardiac Arrest
The risk of rapid infusion may be necessary because of the potential for fatality due to acidosis. Sodium bicarbonate solutions are usually hypertonic and may produce an undesirable rise in plasma sodium concentration in the process of correction of metabolic acidosis. In cardiac arrest, however, the risks from acidosis exceed those of hypernatremia, which can be treated in the next phase of the therapeutic regimen.
Electrolyte/solute overload
The risk of dilutional states is inversely proportional to the electrolyte concentrations of administered parenteral solutions. The risk of solute overload causing congested states with peripheral and pulmonary edema is directly proportional to the electrolyte concentrations of such solutions.
PRECAUTIONS
General
Overdosage and alkalosis should be avoided by giving repeated small doses and monitoring appropriate laboratory tests.
Vascular
The solutions of hypertonic NaHCO3 may cause vascular irritation or sloughing if given extravascularly. Avoid scalp vein use with hypertonic solutions.
Bicarbonate
In the process of substantially correcting the low total CO2 content and blood pH with bicarbonate therapy, the risks of overdosage and alkalosis should be avoided.
Repeated fractional doses and periodic monitoring by suitable laboratory test, e.g., arterial blood gases, are therefore recommended to minimize the possibility of overdosage. The actual total amount of sodium bicarbonate administered is governed by the successive clinical responses of the patient to each repeated fractional dose. Once the severe symptoms have been controlled, the size of each fractional dose and the frequency of administration should be decreased in order to gradually restore the normal bicarbonate levels.
CHF
Since sodium accompanies bicarbonate, caution should be observed in patients with congestive heart failure or other edematous or sodium-retaining states, as well as in patients with oliguria or anuria. Each mL of the following concentrations of sodium bicarbonate injection contains the following corresponding amounts of sodium (Na+):
| 4.2% NaHCO3 = 0.5 | mEq Na+/ mL (11.5 mg Na+/ mL) |
| 8.4% NaHCO3 = 1 | mEq Na+/ mL (22.9 mg Na+/ mL) |
Potassium depletion may predispose to metabolic alkalosis, and coexistent hypocalcemia may be associated with carpopedal spasm as the plasma pH rises. These dangers can be minimized if such electrolyte imbalances are treated prior to or concomitantly with bicarbonate.
Excessive administration of sodium bicarbonate can cause an intracellular shift of potassium, inducing hypokalemia and predisposing the patient to cardiac arrhythmias.
Patients losing chloride by vomiting or gastrointestinal suction are more susceptible to developing severe alkalosis if given alkalinizing agents.
Both pulmonary ventilation and perfusion must be adequately supported whenever respiratory acidosis is concomitant with metabolic acidosis in order to get rid of excess CO2.
DRUG INTERACTIONS
Parenteral Fluid Compatibility
The addition of sodium bicarbonate to parenteral solutions containing calcium should be avoided, except where compatibility has been previously established. Precipitation or haze may result from sodium bicarbonate-calcium admixtures. Should this occur, such a solution should be immediately discarded.
Caution should be observed when giving parenteral fluids, especially those containing sodium ions, to patients receiving corticosteroids or corticotropin.
Increased half-lives and duration of action of quinidine, amphetamines, ephedrine, and pseudoephedrine may occur with urinary alkalinization by sodium bicarbonate.
Sodium bicarbonate, by producing an alkaline urine, increases the renal clearance of tetracyclines, especially doxycycline.
USAGE IN PREGNANCY
Teratogenic Effect
Pregnancy Category C
Animal reproduction studies have not been conducted with sodium bicarbonate. It is also not known whether sodium bicarbonate can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Sodium bicarbonate should be given to a pregnant woman only if clearly needed.
Usage in Impaired Renal Function
Administration of solutions containing sodium ions may result in sodium retention.
ADVERSE REACTIONS
Alkalosis and / or hypokalemia may ensue as a result of overdosage or over-correction of the bicarbonate deficit. Severe alkalosis may be accompanied by hyperirritability or tetany.
Extravasation of I.V. hypertonic solutions of sodium bicarbonate may cause chemical cellulitus, with tissue necrosis, ulceration or sloughing at the site of infiltration. Prompt elevation of the part, warmth and local injection of lidocaine or hyaluronidase are recommended to prevent sloughing.
OVERDOSAGE
Symptoms
Excessive or too rapid administration may produce alkalosis. Severe alkalosis may be accompanied by hyperirritability or tetany. (See ADVERSE REACTIONS).
Treatment
Sodium bicarbonate should be discontinued and the symptoms of alkalosis controlled by rebreathing expired air from a paper bag or rebreathing mask or, if more severe, by parenteral injections of calcium gluconate. Severe alkalosis may be corrected by I.V. infusion of 2.14% ammonium chloride solution, except in patients with hepatic disease, in whom ammonia use is contraindicated. Sodium chloride (0.9%) I.V. or potassium chloride may be indicated if there is hypokalemia. Calcium gluconate is administered to control tetany.
DOSAGE AND ADMINISTRATION
Please note that the optional STICK-GARD® Safety Needle, featured with stock number 2052 is interchangeable with a standard needle.
Sodium Bicarbonate Injection, USP is administered by the intravenous route, preferably into a large vein. Suitable concentrations range from 1.4% (isotonic) to 8.4% (undiluted), depending upon the clinical condition and requirement of the patient. The MIN-I-JET® Luer-Lock disposable syringe features STICK-GARD®, a recessed needle enclosed in a protective plastic sheath. The Stick-Gard® Safety Needle is pre-attached to the Luer-Lock of the syringe injector. The Stick-Gard® Safety Needle is intended for use onto the Y-site of an I.V. set or a heparin lock injection site. Since the Stick-Gard® Safety Needle cannot be used for direct injection (I.V. or I.M.) or for entering glass I.V. bottles and certain plastic I.V. bags, the Stick-Gard® Safety Needle should be replaced with a suitable needle when the above modes of administration are recommended or appropriate.
The need for Sodium Bicarbonate Injection is dependent upon the pH of the serum and the clinical symptoms of the patient as well as the base (bicarbonate) deficit. However, the dose of sodium bicarbonate can be based primarily upon the plasma deficit. This is the difference between the average normal bicarbonate of the plasma (27 mEq/ L) and the value determined for the patient.
If plasma bicarbonate of the patient with metabolic acidosis is unknown, a safe average of sodium bicarbonate is 5 mEq (420 mg) per kilogram of body weight. If the patient has severe metabolic acidosis, bicarbonate solutions containing 90–180 mEq/L (approximately 7.5 – 15 g) may be given at rates of 1–1.5 liters during the first hour. The concentration of bicarbonate solutions used for further management of the patient may be adjusted to the needs of the patient.
Usual adult dosage
Systemic alkalizer —
- In cardiac arrest: Intravenous, initially 1 mEq per kg of body weight; 0.5 mEq per kg of body weight may be repeated every ten minutes of continued arrest.
- In less urgent forms of metabolic acidosis: Intravenous infusion, 2 to 5 mEq per kg of body weight, administered over a period of 4 to 8 hours.
- Note: Frequency of administration and the size of the dose may be reduced after severe symptoms have abated.
Urinary alkalizer — Intravenous, 2 to 5 mEq per kg of body weight, administered over a period of 4 to 8 hours.
Usual pediatric dosage
Systemic alkalizer —
- In cardiac arrest: Intravenous, 1 mEq per kg of body weight initially, then 0.5 mEq per kg body weight every ten minutes of continued arrest.
- In less urgent forms of metabolic acidosis: Older children — See Usual adult dosage.
Urinary alkalizer — See Usual adult dosage.
In cardiac arrest
Sodium bicarbonate should be administered according to the result of arterial blood pH and PaC02 and calculation of base deficit. Caution should be observed where rapid infusion of large quantities of bicarbonate is indicated. Bicarbonate solutions are hypertonic and may produce an undesirable rise in plasma sodium concentration. In cardiac arrest however, the risks from acidosis exceed those of hypernatremia.
In infants, (up to two years of age), the 4.2% solution is recommended for intravenous administration at a rate not to exceed 8 mEq / kg/ day. This dosage is recommended in neonates to guard against the possibility of producing hypernatremia, decreasing cerebrospinal fluid pressure and inducing intracranial hemorrhage.
If base deficit is known, a calculated dose of 0.3 × kg × base deficit is given. If only 8.4% sodium bicarbonate is available, it may be diluted 1:1 with 5% dextrose in water before administration.
In less urgent forms of metabolic acidosis
Sodium Bicarbonate Injection, USP, may be added to other intravenous fluids. Desired dilutions may be prepared using sterile water, sodium chloride (0.9%), dextrose 5%, or other standard electrolyte solutions as diluent. The amount of bicarbonate to be given to older children and adults over a four-to eight-hour period depends upon the severity of the acidosis as judged by the lowering of total C02 content, blood pH and clinical condition of the patient. Bicarbonate therapy should always be planned in a stepwise fashion since the degree of response from a given dose is not precisely predicable. Initially an infusion of 2 to 5 mEq per kg body weight over a period of 4 to 8 hours will produce a measureable improvement in the abnormal acid-base status of the blood.
Alternatively, estimates of the initial dose of sodium bicarbonate may be based on the following equation:
0.5 × body weight (kg) × desired increase in serum HCO3- (mEq / L) = bicarbonate dose (mEq) or
0.5 × body weight (kg) × base deficit (mEq / L)= bicarbonate dose (mEq).
One-half of this calculated estimate is given. The next step of therapy is dependent upon the clinical response of the patient. If severe symptoms have abated, the frequency of administration and dose may be reduced.
In general, it is unwise to attempt full correction of a low total CO2 content during the first 24 hours of therapy, since this may be accompanied by an unrecognized alkalosis because of a delay in the readjustment of ventilation to normal. Owing to this lag, the achievement of total CO2 content of about 20 mEq/ L at the end of the first day of therapy will usually be associated with a normal blood pH. Further modification of the acidosis to completely normal values usually occurs in the presence of normal kidney function when and if the cause of the acidosis can be controlled. Values for total CO2 which are brought to normal or above normal within the first day of therapy are very likely to be associated with grossly alkaline values for blood pH, with ensuing undesirable side effects.
NOTE: Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Do not use the injection if it contains a precipitate.
HOW SUPPLIED
Sodium Bicarbonate Injection, USP
In unit-use packages containing a MIN-I-JET® disposable syringe.
| Stock No. | Size and Type | Concentration | NDC No. |
|---|---|---|---|
| 1052 | 50 mL with 18 G. × 11/2" needle | 8.4% | 0548-1052-00 |
In unit-use packages containing a MIN-I-JET® disposable syringe with Luer-Lock and optional STICK-GARD® Safety Needle.
| Stock No. | Size and Type | Concentration | NDC No. |
|---|---|---|---|
| 2052 | 50 mL with STICK-GARD® | 8.4% | 0548-2052-00 |
Twenty-five unit-use packages per carton.
Manufactured under U.S. Pat No. 4,834,716, Reissue No 33,617, STICK-GARD® Safety Needle.
In unit-use packages containing a Luer-Jet™ Luer-Lock Prefilled Syringe.
| Stock No. | Size and Type | Concentration | NDC No. |
|---|---|---|---|
| 3331 | 10 mL with LuerJet™ Luer-Lock | 4.2% | 0548-3331-00 |
| 3352 | 50 mL with LuerJet™ Luer-Lock | 8.4% | 0548-3352-00 |
Ten unit-use packages per carton.
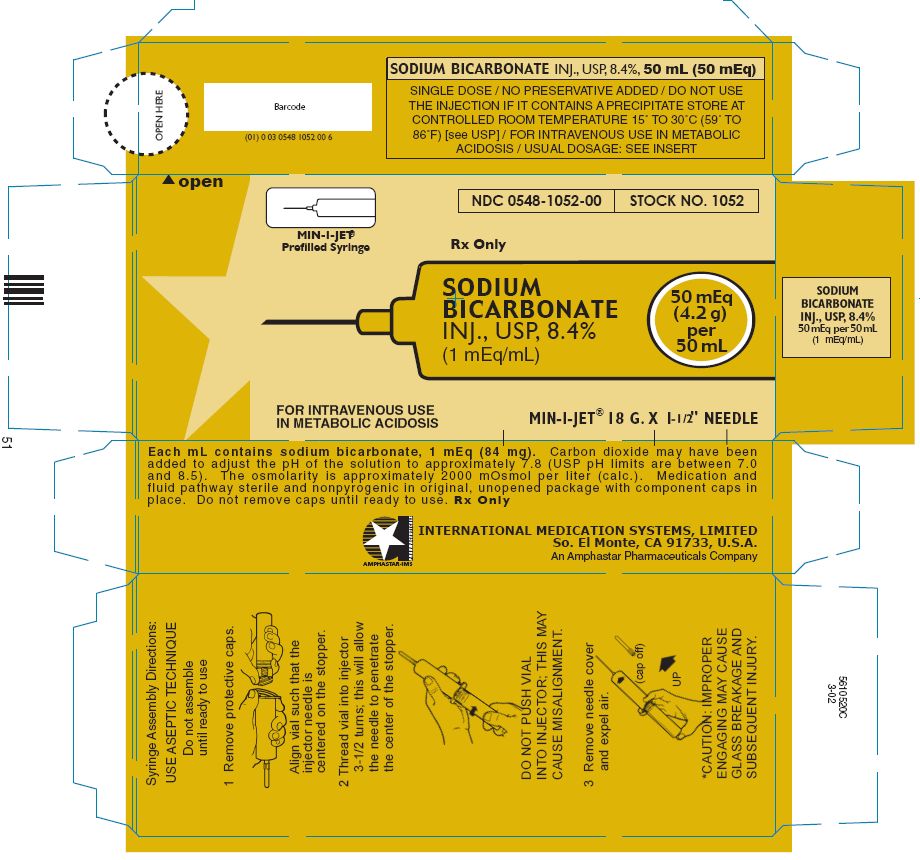
Syringe Assembly Directions
The MIN-I-JET® syringe with needle, illustrated below, is the basic unit upon which all the other syringe systems are built; slight adaptations and/ or additional auxillary parts create the other syringe systems. Assembly directions remain essentially the same.
USE ASEPTIC TECHNIQUE
Do not assemble until ready to use.
|
||
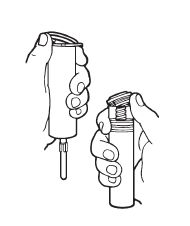 Remove protective caps. Align vial such that the injector needle is centered on the stopper. | 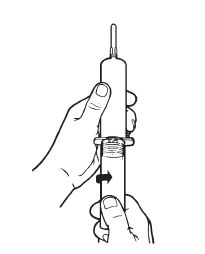 Thread vial into injector 3 half turns, or until needle penetrates stopper.* DO NOT PUSH VIAL INTO INJECTOR; THIS MAY CAUSE MISALIGNMENT. | 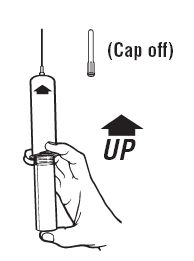 Remove needle cap and expel air. |
Store at controlled room temperature 15° to 30°C (59° to 86°F).
Rx Only
INTERNATIONAL MEDICATION SYSTEMS, LIMITED
SO. EL MONTE, CA 91733, U.S.A.
An Amphastar Pharmaceuticals Company
Rev. 1-08
6920020J
PRINCIPAL DISPLAY PANEL - 4.2 g per 50 mL MIN-I-JET Prefilled Syringe label
MIN-I-JET®
Prefilled Syringe
NDC 0548-1052-00
STOCK NO. 1052
Rx Only
SODIUM
BICARBONATE
INJ., USP, 8.4%
(1 mEq/mL)
50 mEq
(4.2 g)
per
50 mL
FOR INTRAVENOUS USE
IN METABOLIC ACIDOSIS
MIN-I-JET® 18 G. X 1 - 1 / 2" NEEDLE
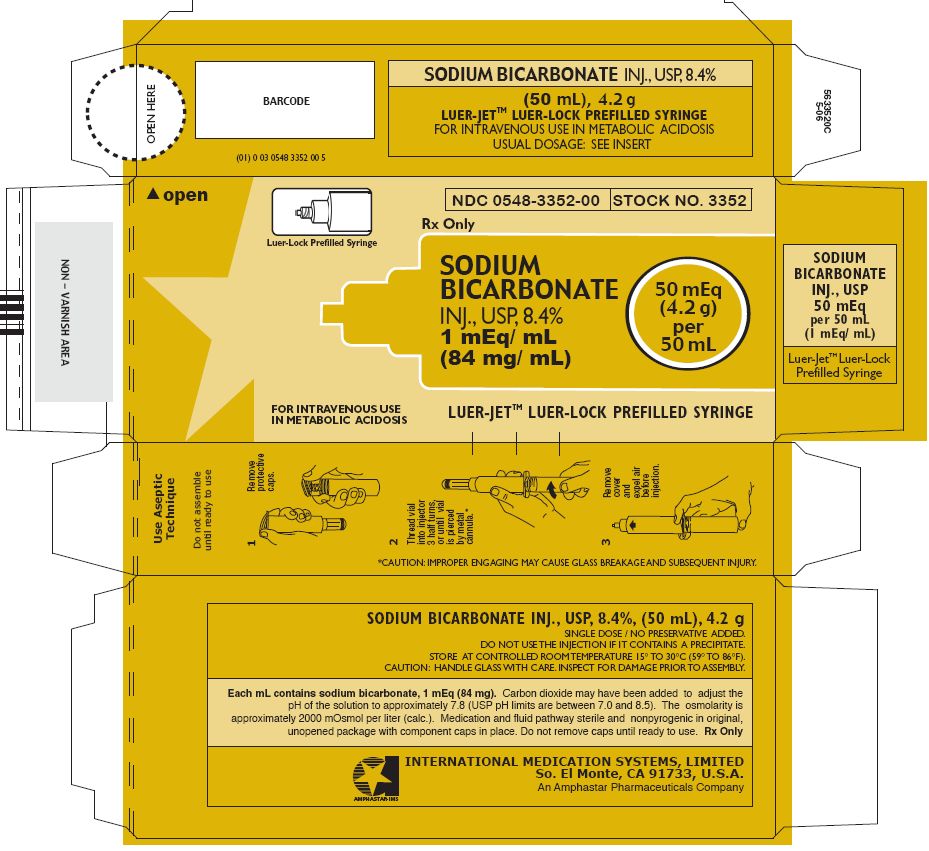
PRINCIPAL DISPLAY PANEL - 4.2 g per 50 mL STICK-GARD Prefilled Syringe label
FEATURING STICK-GARD®
18 G. SAFETY INJECTOR
NDC 0548-2052-00
STOCK NO. 2052
Rx Only
SODIUM
BICARBONATE
INJ., USP, 8.4%
(1 mEq/mL)
50 mEq
(4.2 g)
per 50 mL
1 mEq/mL
(84 mg/mL)
FOR INTRAVENOUS USE
IN METABOLIC ACIDOSIS
STICK-GARD® PREFILLED SYRINGE
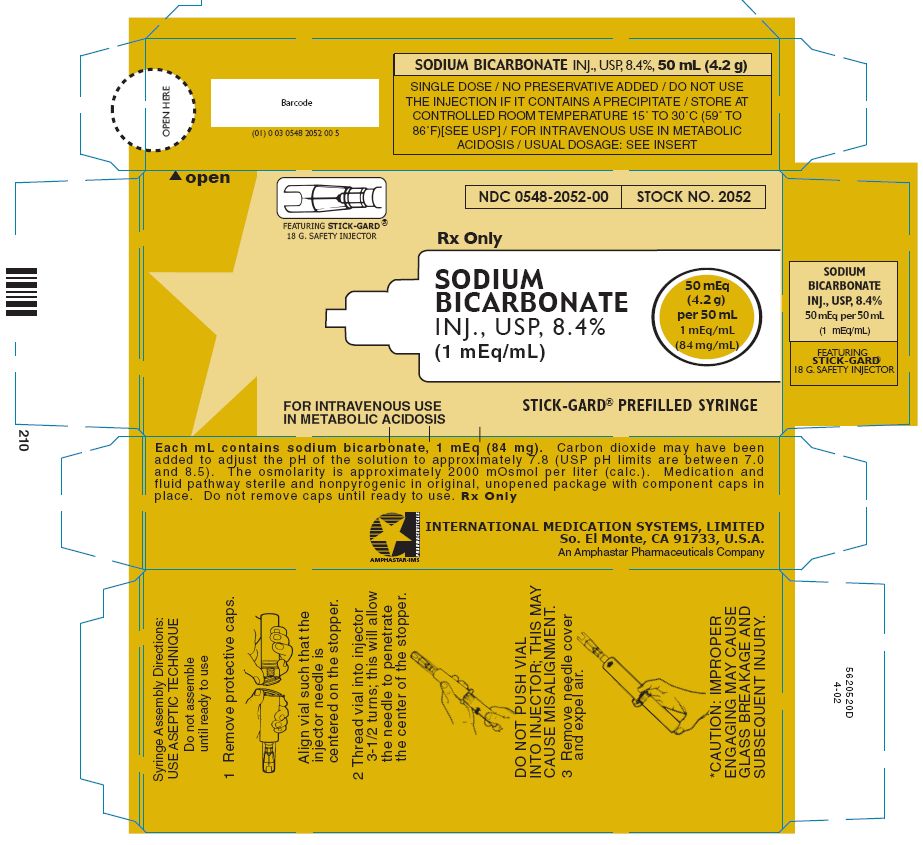
PRINCIPAL DISPLAY PANEL - 0.42 g per 10 mL LUER-LOCK Prefilled Syringe label
Luer-Lock Prefilled Syringe
NDC 0548-3331-00
STOCK NO. 3331
Rx only
SODIUM
BICARBONATE
INJ., USP, 4.2%
0.5 mEq/ mL
(42 mg/ mL)
5 mEq
(0.42 g)
per
10 mL
FOR INTRAVENOUS USE
IN METABOLIC ACIDOSIS
PEDIATRIC SPECIALTY
LUER-JET™ LUER-LOCK PREFILLED SYRINGE
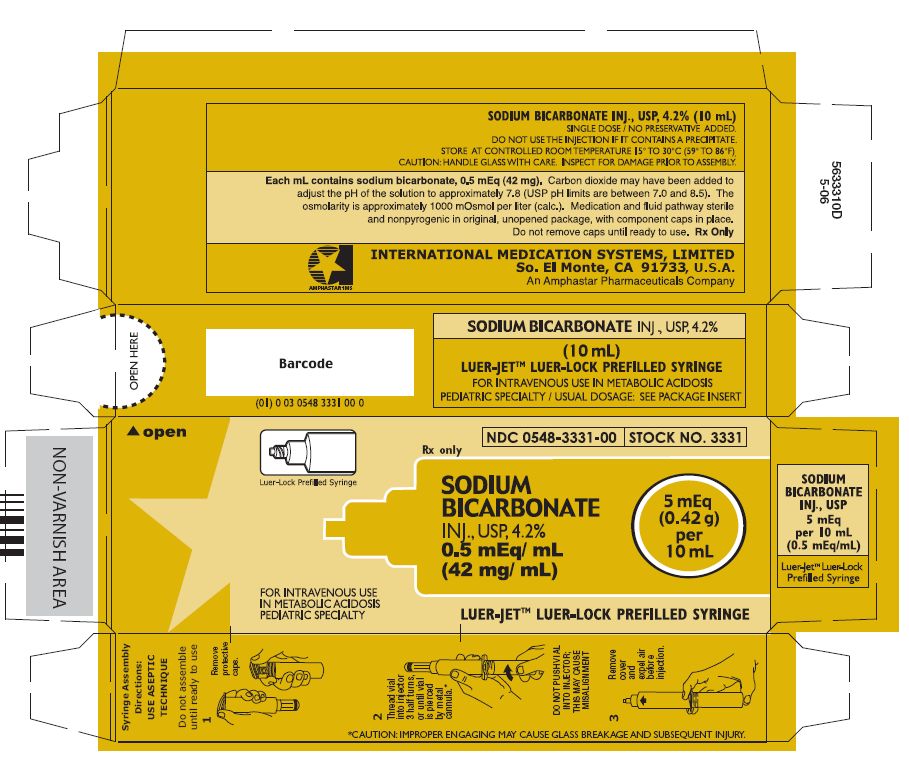
PRINCIPAL DISPLAY PANEL - 4.2 g per 50 mL LUER-LOCK Prefilled Syringe label
Luer-Lock Prefilled Syringe
NDC 0548-3352-00
STOCK NO. 3352
Rx Only
SODIUM
BICARBONATE
INJ., USP, 8.4%
1 mEq/ mL
(84 mg/ mL)
50 mEq
(4.2 g)
per
50 mL
FOR INTRAVENOUS USE
IN METABOLIC ACIDOSIS
LUER-JET™ LUER-LOCK PREFILLED SYRINGE
| SODIUM BICARBONATE
sodium bicarbonate injection |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| UNAPPROVED DRUG OTHER | 04/01/1973 | ||
| SODIUM BICARBONATE
sodium bicarbonate injection |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| UNAPPROVED DRUG OTHER | 01/02/1990 | ||
| SODIUM BICARBONATE
sodium bicarbonate injection |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| UNAPPROVED DRUG OTHER | 06/01/2000 | ||
| SODIUM BICARBONATE
sodium bicarbonate injection |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| UNAPPROVED DRUG OTHER | 06/01/2000 | ||
| Labeler - Amphastar Pharmaceuticals, Inc. (024736733) |