HYDRO EAR DROPS
-
hydrocortisone,
pramoxine hydrochloride,
chloroxylenol and
benzalkonium chloride solution/ drops
Hi-Tech Pharmacal Co., Inc.
----------
Hydro Ear DropsDESCRIPTION
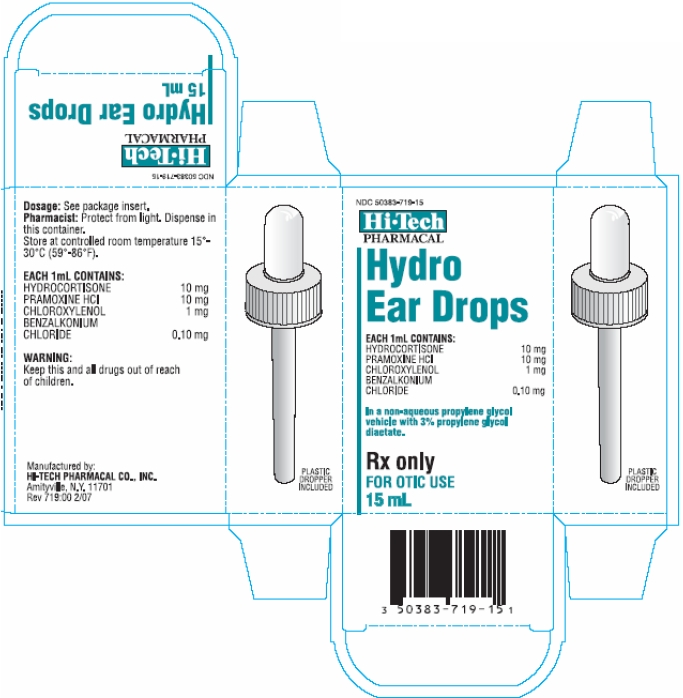
Each 1 mL for otic administration contains:
Hydrocortisone 10 mg
Pramoxine Hydrochloride 10 mg
Chloroxylenol 1 mg
Benzalkonium Chloride 0.10 mg
In a non-aqueous propylene glycol vehicle with 3% propylene glycol diacetate.
Chloroxylenol:
4-chloro-3.5 dimethylphenol is a broad spectrum bactericidal agent which occurs as white crystals with a phenolic odor. It is soluble in alcohol, ether, and alkali hydroxides.
Hydrocortisone:
11, 17, 21, trihydroxy, (11 b)-Pregn-4-ene-3,20-dione, is an anti-inflammatory and antipruritic agent. It occurs as a white to practically white, odorless, crystalline powder which melts at about 215 °C with decomposition. It is very slightly soluble in water and in ether; sparingly soluble in acetone and in alcohol; slightly soluble in chloroform.
Pramoxine hydrochloride:
4[3-(4-butoxyphenoxy) propyl] - Morpholine hydrochloride is a topical anesthetic proven to be safe and effective in hospital tests. It occurs as a white to practically white, crystalline powder, having a numbing taste and it may have a slight aromatic odor. The pH of a solution (1 in 100) is about 4.5, it is freely soluble in water and in alcohol; soluble in chloroform; and very slightly soluble in ether.
Benzalkonium chloride:
Action lowers surface tension permitting greater penetration to meatal skin. It also is a cationic germicide of high bactericidal and bacteriostatic potency.
CLINICAL PHARMACOLOGY
This product is effective both as an antibacterial and antifungal agent. The special base is a hydrophilic solution having an acid pH and a low surface tension, exerting a drying effect and allowing the medication to spread quickly to all contiguous surfaces, softening and reducing accumulated cerumen.
Chloroxylenol is a halogenated phenol, non-toxic, non-corrosive, non-staining with a high phenol coefficient. It may be applied directly to a wound and shows no chemical reactivity toward blood.
Hydrocortisone is a topical corticosteroid which has anti-inflammatory, antipruritic and vasoconstrictive actions. The mechanism of anti-inflammatory activity of the corticosteroids is unclear. Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin increase percutaneous absorption. Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids. Corticosteroids are bound to plasma proteins in varying degrees. Corticosteroids are metabolized primarily in the liver and are then excreted by the kidneys. Some of the topical corticosteroids and their metabolites are also excreted into the bile.
Pramoxine hydrochloride is a topical anesthetic agent which provides temporary relief from itching and pain. It acts by stabilizing the neuronal membranes of nerve endings with which it comes into contact.
INDICATIONS AND USAGE
For the treatment of superficial infections of the external auditory canal complicated by inflammation caused by organisms and susceptible to the action of the antimicrobial. May also be used to control itching in the auditory canal.
CONTRAINDICATIONS
Topical steroids are contraindicated in varicella, vaccinia and patients sensitive to any of the components of this preparation. This or any medication should not be applied in the external auditory canal of patients with perforated eardrums.
WARNINGS
This preparation is not intended for ophthalmic or oral use. If accidental ingestion occurs, seek professional help. If irritation or sensitization occurs, promptly discontinue use of this preparation and institute other measures.
If irritation or sensitization occurs, promptly discontinue use of this preparation and institute other measures.
PRECAUTIONS
General
If a favorable response does not occur promptly, discontinue the use of this preparation until the infection is controlled by other appropriate measures. Although systemic side effects are not common with topical corticosteroids, the possibility of occurrence must be kept in mind particularly when used for an extended period of time.
Systemic absorption of topical corticosteroids has produced reversible hypothalamic-pituitary-adrenal (HPA) axis suppression, manifestations of Cushing’s syndrome, hyperglycemia and glycosuria in some patients. Conditions which augment systemic absorption include the application of more potent steroids. Recovery of HPA axis function is generally prompt and complete upon discontinuation of the drug. Infrequently, signs and symptoms of steroid withdrawal may occur, requiring supplemental corticosteroids. Children may absorb proportionately larger amounts of topical corticosteroids and thus be more susceptible to systemic toxicity (see PRECAUTIONS - Pediatric use).
Information for patients
Patients using topical corticosteroids should receive the following information and instructions:
- This medication is to be used as directed by the physician. It is for external use only. Avoid contact with the eyes.
- Patients should be advised not to use this medication for any disorder other than for which it was prescribed.
- Patients should report any signs of local adverse reactions.
Laboratory tests
The urinary free cortisol test and the ACTH stimulation test may be helpful in evaluating the HPA axis suppression.
Carcinogenesis, mutagenesis, impairment of fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential or the effect of topical corticosteroids on fertility. Studies to determine mutagenicity with prednisone and hydrocortisone have revealed negative results.
Pregnancy
Pregnancy Category C: Corticosteroids are generally teratogenic in laboratory animals when administered systemically at low dosage levels. The more potent corticosteroids have been shown to be teratogenic after dermal application in laboratory animals. There are no adequate and well controlled studies in pregnant women on teratogenic effects from topically applied corticosteroids. Therefore, topical corticosteroids should be used during pregnancy only if the benefit justifies the potential risk to the fetus. Drugs of this class should not be used extensively on pregnant patients in large amounts, or for prolonged periods of time.
Nursing mothers
It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in breast milk. Systemically administered corticosteroids are secreted into breast milk in quantities not likely to have a deleterious effect on the infant. Nevertheless, caution should be exercised when topical corticosteroids are administered to a nursing woman.
Pediatric use
Pediatric patients may demonstrate greater susceptibility to topical corticosteroid-induced Hypothalmic-pituitary-adrenal axis suppression and Cushing’s syndrome than mature patients because of a larger skin surface to body weight ratio. HPA axis suppression, Cushing’s syndrome and intracranial hypertension have been reported in children receiving topical corticosteroids. Manifestations of adrenal suppression in children include linear growth retardation, delayed weight gain, low plasma cortisol levels and absence of response to ACTH stimulation. Manifestation of intracranial hypertension include bulging fontanelles, headaches and bilateral papilledema. Administration of topical corticosteroids to children should be limited to the least amount compatible with an effective therapeutic regimen. Chronic corticosteroid therapy may interfere with the growth and development of children.
Drug Interactions
Interaction of other drugs with the hydrocortisone component of this product is possible. Inform your physician of other medication you are currently taking.
ADVERSE REACTIONS
The following adverse reactions with topical corticosteroids have been observed: itching, burning, irritation, dryness, folliculitis, hypertrichosis, acneform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, maceration of the skin, secondary infection, skin atrophy, striae and miliaria.
OVERDOSAGE
Topically applied corticosteroids can be absorbed in sufficient amount to produce systemic effects.
DOSAGE AND ADMINISTRATION
The external auditory canal should be thoroughly cleansed and dried with a sterile cotton applicator. For adults, 4 to 5 drops of the solution should be instilled into the affected ear 3 or 4 times daily. For infants and small children, 3 drops are suggested because of the smaller capacity of the ear canal. The patient should lie with the affected ear upward to instill the drops and this position maintained for 5 minutes to facilitate penetration of the drops into the ear canal. Repeat, if necessary, for the opposite ear.
HOW SUPPLIED
Hydrocortisone, Pramoxine HCl, Chloroxylenol and Benzalkonium Chloride Ear Drops are supplied in 15ml amber glass bottles with dosage dropper.
Storage
Store at controlled room temperature 15°-30°C (59°-86°F). Protect from light. Dispense in original container.
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN.
Rx Only
Mfg. by
Hi-Tech Pharmacal Co., Inc.,
Amityville, NY 11701
Rev 719:00 07/05
MG #21467
PRINCIPAL DISPLAY PANEL
Container Label

Carton
| HYDRO EAR DROPS
hydrocortisone, pramoxine hcl, chloroxylenol and benzalkonium chloride ear drops solution/ drops |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Unapproved other | 08/01/2002 | ||
| Labeler - Hi-Tech Pharmacal Co., Inc. (101196749) |