FANATREX
-
gabapentin
Fusion Pharmaceuticals LLC
----------
FANATREXDESCRIPTION
NDC 43093-105-01
Rx only
FusePaq™
FANATREX™
(gabapentin 25 mg/mL, in oral suspension - kit)
FusePaq™ kits provide a convenient approach to rapidly prepare prescription medications, as all components are pre-measured. This kit is manufactured according to US FDA current Good Manufacturing Practices (cGMP).
Description:
This kit contains active and inactive bulk materials to prepare 420 mL of a gabapentin oral suspension containing 25 mg/mL gabapentin. This kit may only be used for the extemporaneous mixing of these ingredients by an appropriate licensed medical professional, in response to a physician's prescription, to create a medication tailored to the specialized needs of an individual patient.
Contents:
- 10.5 g gabapentin, USP
- 420 mL oral suspension vehicle (water, banana flavor, N-acetyl-D-glucosamine, strawberry flavor, marshmallow flavor, glycerin, stevia powder, acesulfame potassium, xanthan gum, monoammonium glycyrrhizinate, sodium saccharin, sodium benzoate, potassium sorbate, dibasic sodium phosphate)
- Disposable funnel
- Press-in bottle adaptor for oral dispenser
- Oral dispenser
- Instructions
SUGGESTED PREPARATION
Suggested Preparation
Gabapentin, 25 mg/mL oral suspension
1 Remove and Inspect the Contents of the Kit
Remove kit contents. Ensure that seals are present and intact on the gabapentin and oral suspension vehicle bottles. If the seals are not intact, do not use the kit.
2 Prepare for Mixing
Wear gloves and eye protection during mixing operations. Remove the seal from the oral suspension bottle. Break the seal and remove the cap from the gabapentin bottle.
3 Transfer Gabapentin to the Suspension Bottle
Uncap the suspension bottle. Using the included funnel, carefully transfer the gabapentin powder to the suspension bottle. Cap the suspension bottle and mix thoroughly by inverting and shaking until all contents are dissolved. Uncap the suspension bottle. Pour a small amount of the mixed suspension back into the gabapentin bottle. Cap the gabapentin bottle and shake to ensure that all residual gabapentin has been dissolved. Pour the liquid through the funnel into the suspension bottle. Discard the funnel and gabapentin powder bottle.
4 Complete the Mixing Process
Insert the press-in bottle adaptor into the suspension bottle. Recap the suspension bottle. Mix well by inverting repeatedly several times. Visually ensure that all contents are dissolved.
5 Re-label the Suspension
Label the mixed suspension as required for prescription products. Ensure that the original oral suspension vehicle label is removed or obscured, since the original label is no longer accurate once the suspension is prepared.
Store the unused kit at room temperature of 15-30°C (59-86°F). Once prepared, store the mixed suspension between 15-30°C (59-86°F). The mixed suspension is stable for at least eight weeks, based upon real-time and accelerated stability studies.
Each lot of suspension vehicle is tested to meet microbial limits per USP Microbial Limit Test <61>. In addition, the suspension vehicle formulation has passed the USP <51> Antimicrobial Effectiveness Test.
An oral dispenser is provided in the kit and may be used to facilitate delivery of the suspension.
U.S. Patents Pending
Manufactured by:
Fusion Pharmaceuticals, LLC
768 Calle Plano
Camarillo, CA 93012
CS75-A1 rev 0
DRUG BOTTLE LABEL
Do not use if safety seal is broken
Gabapentin
1-(Aminomethyl)cyclohexaneacetic acid
CAS #60142-96-3
CAUTION: For manufacturing, processing, repacking, or prescription compounding
Net contents: 10.5 g
Repackaged by Fusion Pharmaceuticals, LLC
Camarillo, CA 93012
CS73-A1 rev 0

SUSPENSION BOTTLE LABEL
Do not use if safety seal is broken
For Prescription Compounding Only
Oral Suspension Vehicle
Dye and paraben free
Ingredients: water, banana flavor, N-acetyl-D-glucosamine, strawberry flavor, marshmallow flavor, glycerin, stevia powder, acesulfame potassium, xanthan gum, monoammonium glycyrrhizinate, sodium saccharin, potassium sorbate, sodium benzoate, dibasic sodium phosphate
Net Contents: 420 mL (14.2 fl oz)
Manufactured for:
Fusion Pharmaceuticals, LLC
Camarillo, CA 93012
CS74-A1 rev 0

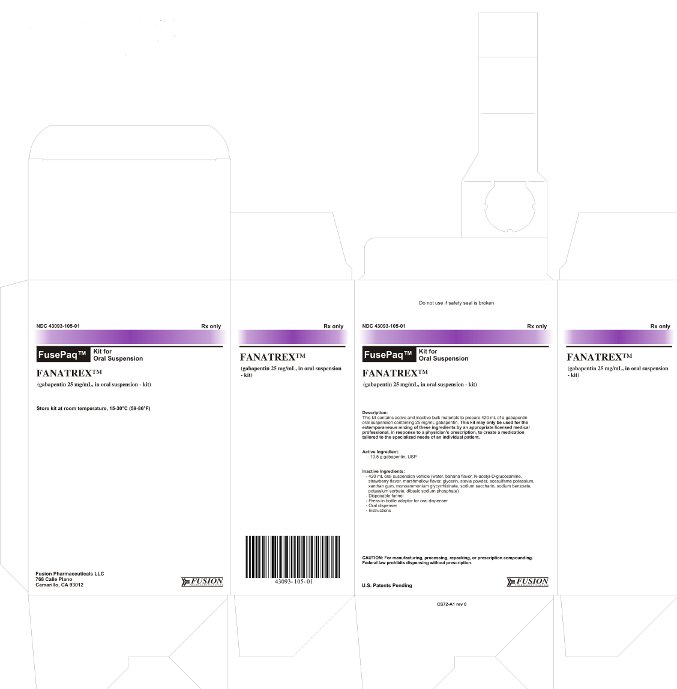
Carton Box Label
Do not use if safety seal is broken
NDC 43093-105-01
Rx only
FusePaq™ Kit for Oral Suspension
Fanatrex™
(gabapentin 25 mg/mL, in oral suspension - kit)
Description:
This kit contains active and inactive bulk materials to prepare 420 mL of a gabapentin oral suspension containing 25 mg/mL gabapentin. This kit may only be used for the extemporaneous mixing of these ingredients by an appropriate licensed medical professional, in response to a physician's prescription, to create a medication tailored to the specialized needs of an individual patient.
Active Ingredient:
- 10.5 g gabapentin, USP
Inactive Ingredients:
- 420 mL oral suspension vehicle (water, banana flavor, N-acetyl-D-glucosamine, strawberry flavor, marshmallow flavor, glycerin, stevia powder, acesulfame potassium, xanthan gum, monoammonium glycyrrhizinate, sodium saccharin, potassium sorbate, sodium benzoate, dibasic sodium phosphate)
- Disposable funnel
- Press-in bottle adaptor for oral dispenser
- Oral dispenser
- Instructions
CAUTION: For manufacturing, processing, repacking, or presciption compounding. Federal law prohibits dispensing without prescription.
U.S. Patents Pending
CS72-A1 rev 0
| FANATREX
gabapentin kit |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved drug other | 05/15/2010 | ||
| Labeler - Fusion Pharmaceuticals LLC (021420944) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Fusion Pharmaceuticals LLC | 021420944 | manufacture | |