delestrogen (estradiol valerate) injection
[Monarch Pharmaceuticals, Inc.]
WARNINGS
-
ESTROGENS HAVE BEEN REPORTED TO INCREASE THE RISK OF ENDOMETRIAL CARCINOMA IN POSTMENOPAUSAL WOMEN.
Close clinical surveillance of all women taking estrogens is important. Adequate diagnostic measures, including endometrial sampling when indicated, should be undertaken to rule out malignancy in all cases of undiagnosed persistent or recurring abnormal vaginal bleeding. There is no evidence that “natural” estrogens are more or less hazardous than “synthetic” estrogens at equiestrogenic doses.
-
ESTROGENS SHOULD NOT BE USED DURING PREGNANCY.
There is no indication for estrogen therapy during pregnancy or during the immediate postpartum period. Estrogens are ineffective for the prevention or treatment of threatened or habitual abortion. Estrogens are not indicated for the prevention of postpartum breast engorgement.
Estrogen therapy during pregnancy is associated with an increased risk of congenital defects in the reproductive organs of the fetus, and possibly other birth defects. Studies of women who received diethylstilbestrol (DES) during pregnancy have shown that female offspring have an increased risk of vaginal adenosis, squamous cell dysplasia of the uterine cervix, and clear cell vaginal cancer later in life; male offspring have an increased risk of urogenital abnormalities and possibly testicular cancer later in life. The 1985 DES Task Force concluded that use of DES during pregnancy is associated with a subsequent increased risk of breast cancer in the mothers, although a causal relationship remains unproven and the observed level of excess risk is similar to that for a number of other breast cancer risk factors.
Description
DELESTROGEN® (estradiol valerate injection, USP) contains estradiol valerate, a long-acting estrogen in sterile oil solutions for intramuscular use. These solutions are clear, colorless to pale yellow. Formulations (per mL): 10 mg estradiol valerate in a vehicle containing 5 mg chlorobutanol (chloral derivative/preservative) and sesame oil; 20 mg estradiol valerate in a vehicle containing 224 mg benzyl benzoate, 20 mg benzyl alcohol (preservative), and castor oil; 40 mg estradiol valerate in a vehicle containing 447 mg benzyl benzoate, 20 mg benzyl alcohol, and castor oil.
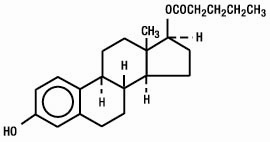
Estradiol valerate is designated chemically as estra-1,3,5(10)-triene-3, 17-diol(17β)-, 17-pentanoate. Graphic formula:
Clinical Pharmacology
Estrogen drug products act by regulating the transcription of a limited number of genes. Estrogens diffuse through cell membranes, distribute themselves throughout the cell, and bind to and activate the nuclear estrogen receptor, a DNA-binding protein which is found in estrogen-responsive tissues. The activated estrogen receptor binds to specific DNA sequences, or hormone-response elements, which enhance the transcription of adjacent genes and in turn lead to the observed effects. Estrogen receptors have been identified in tissues of the reproductive tract, breast, pituitary, hypothalamus, liver, and bone of women.
Estrogens are important in the development and maintenance of the female reproductive system and secondary sex characteristics. By a direct action, they cause growth and development of the uterus, fallopian tubes, and vagina. With other hormones, such as pituitary hormones and progesterone, they cause enlargement of the breasts through promotion of ductal growth, stromal development, and the accretion of fat. Estrogens are intricately involved with other hormones, especially progesterone, in the processes of the ovulatory menstrual cycle and pregnancy, and affect the release of pituitary gonadotropins. They also contribute to the shaping of the skeleton, maintenance of tone and elasticity of urogenital structures, changes in the epiphyses of the long bones that allow for the pubertal growth spurt and its termination, and pigmentation of the nipples and genitals.
Estrogens occur naturally in several forms. The primary source of estrogen in normally cycling adult women is the ovarian follicle, which secretes 70 to 500 micrograms of estradiol daily, depending on the phase of the menstrual cycle. This is converted primarily to estrone, which circulates in roughly equal proportion to estradiol, and to small amounts of estriol. After menopause, most endogenous estrogen is produced by conversion of androstenedione, secreted by the adrenal cortex, to estrone by peripheral tissues. Thus, estrone—especially in its sulfate ester form—is the most abundant circulating estrogen in postmenopausal women. Although circulating estrogens exist in a dynamic equilibrium of metabolic interconversions, estradiol is the principal intracellular human estrogen and is substantially more potent than estrone or estriol at the receptor.
Estrogens used in therapy are well absorbed through the skin, mucous membranes, and gastrointestinal tract. When applied for a local action, absorption is usually sufficient to cause systemic effects. When conjugated with aryl and alkyl groups for parenteral administration, the rate of absorption of oily preparations is slowed with a prolonged duration of action, such that a single intramuscular injection of estradiol valerate or estradiol cypionate is absorbed over several weeks.
Administered estrogens and their esters are handled within the body essentially the same as the endogenous hormones. Metabolic conversion of estrogens occurs primarily in the liver (first pass effect), but also at local target tissue sites. Complex metabolic processes result in a dynamic equilibrium of circulating conjugated and unconjugated estrogenic forms which are continually interconverted, especially between estrone and estradiol and between esterified and non-esterified forms. Although naturally-occurring estrogens circulate in the blood largely bound to sex hormone- binding globulin and albumin, only unbound estrogens enter target tissue cells. A significant proportion of the circulating estrogen exists as sulfate conjugates, especially estrone sulfate, which serves as a circulating reservoir for the formation of more active estrogenic species. A certain proportion of the estrogen is excreted into the bile and then reabsorbed from the intestine. During this enterohepatic recirculation, estrogens are desulfated and resulfated and undergo degradation through conversion to less active estrogens (estriol and other estrogens), oxidation to nonestrogenic substances (catecholestrogens, which interact with catecholamine metabolism, especially in the central nervous system), and conjugation with glucuronic acids (which are then rapidly excreted in the urine).
When given orally, naturally-occurring estrogens and their esters are extensively metabolized (first pass effect) and circulate primarily as estrone sulfate, with smaller amounts of other conjugated and unconjugated estrogenic species. This results in limited oral potency. By contrast, synthetic estrogens, such as ethinyl estradiol and the nonsteroidal estrogens, are degraded very slowly in the liver and other tissues, which results in their high intrinsic potency. Estrogen drug products administered by non-oral routes are not subject to first-pass metabolism, but also undergo significant hepatic uptake, metabolism, and enterohepatic recycling.
Indications and Usage
DELESTROGEN (estradiol valerate injection, USP) is indicated in the:
-
Treatment of moderate to severe vasomotor symptoms associated with the menopause. There is no adequate evidence that estrogens are effective for nervous symptoms or depression which might occur during menopause and they should not be used to treat these conditions.
-
Treatment of vulval and vaginal atrophy.
-
Treatment of hypoestrogenism due to hypogonadism, castration or primary ovarian failure.
-
Treatment of advanced androgen-dependent carcinoma of the prostate (for palliation only).
Contraindications
Estrogens should not be used in individuals with any of the following conditions:
-
Known or suspected pregnancy (see Boxed WARNINGS). Estrogens may cause fetal harm when administered to a pregnant woman.
-
Undiagnosed abnormal genital bleeding.
-
Known or suspected cancer of the breast except in appropriately selected patients being treated for metastatic disease.
-
Known or suspected estrogen-dependent neoplasia.
-
Active thrombophlebitis or thromboembolic disorders.
Warnings
-
Induction of malignant neoplasms.Endometrial cancer. The reported endometrial cancer risk among unopposed estrogen users is about 2 to 12 fold greater than in nonusers, and appears dependent on duration of treatment and on estrogen dose. Most studies show no significant increased risk associated with use of estrogens for less than one year. The greatest risk appears associated with prolonged use—with increased risks of 15 to 24-fold for five to ten years or more. In three studies, persistence of risk was demonstrated for 8 to over 15 years after cessation of estrogen treatment. In one study a significant decrease in the incidence of endometrial cancer occurred six months after estrogen withdrawal. Concurrent progestin therapy may offset this risk but the overall health impact in postmenopausal women is not known.
Breast Cancer. While some epidemiologic studies suggest a very modest increase in breast cancer risk for estrogen alone users versus nonusers, other studies have not shown any increased risk. The addition of progestin to estrogen may increase the risk for breast cancer over that noted in non-hormone users more significantly (by about 24-40%), although this is based solely on epidemiologic studies, and definitive conclusions await prospective, controlled clinical trials.
Women without a uterus who require hormone replacement should receive estrogen-alone therapy, and should not be exposed unnecessarily to progestins. Women with a uterus who are candidates for short-term combination estrogen/progestin therapy (for relief of vasomotor symptoms) are not felt to be at a substantially increased risk for breast cancer. Women with a uterus who are candidates for long-term use of estrogen/progestin therapy should be advised of potential benefits and risks (including the potential for increased risk of breast cancer). All women should receive yearly breast exams by a health care provider and perform monthly breast-self examinations. In addition, mammography examinations should be scheduled as suggested by providers based on patient age and risk factors.
Congenital lesions with malignant potential. Estrogen therapy during pregnancy is associated with an increased risk of fetal congenital reproductive tract disorders, and possibly other birth defects. Studies of women who received DES during pregnancy have shown that female offspring have an increased risk of vaginal adenosis, squamous cell dysplasia of the uterine cervix, and clear cell vaginal cancer later in life; male offspring have an increased risk of urogenital abnormalities and possibly testicular cancer later in life. Although some of these changes are benign, others are precursors of malignancy.
-
Gallbladder disease. Two studies have reported a 2- to 4-fold increase in the risk of gallbladder disease requiring surgery in women receiving postmenopausal estrogens.
-
Cardiovascular disease. Large doses of estrogen (5 mg conjugated estrogens per day), comparable to those used to treat cancer of the prostate and breast, have been shown in a large prospective clinical trial in men to increase the risks of nonfatal myocardial infarction, pulmonary embolism, and thrombophlebitis. These risks cannot necessarily be extrapolated from men to women. However, to avoid the theoretical cardiovascular risk to women caused by high estrogen doses, the dose for estrogen replacement therapy should not exceed the lowest effective dose.
-
Elevated blood pressure. Occasional blood pressure increases during estrogen replacement therapy have been attributed to idiosyncratic reactions to estrogens. More often, blood pressure has remained the same or has dropped. One study showed that postmenopausal estrogen users have higher blood pressure than nonusers. Two other studies showed slightly lower blood pressure among estrogen users compared to nonusers. Postmenopausal estrogen use does not increase the risk of stroke. Nonetheless, blood pressure should be monitored at regular intervals with estrogen use.
-
Hypercalcemia. Administration of estrogens may lead to severe hypercalcemia in patients with breast cancer and bone metastases. If this occurs, the drug should be stopped and appropriate measures taken to reduce the serum calcium level.
Precautions
A. General
-
Addition of a progestin. Studies of the addition of a progestin for 10 or more days of a cycle of estrogen administration have reported a lowered incidence of endometrial hyperplasia than would be induced by estrogen treatment alone. Morphological and biochemical studies of endometria suggest that 10 to 14 days of progestin are needed to provide maximal maturation of the endometrium and to reduce the likelihood of hyperplastic changes. There are, however, possible risks which may be associated with the use of progestins in estrogen replacement regimens. These include: (1) adverse effects on lipoprotein metabolism (lowering HDL and raising LDL) which could diminish the purported cardioprotective effect of estrogen therapy (see PRECAUTIONS); (2) impairment of glucose tolerance; and (3) possible enhancement of mitotic activity in breast epithelial tissue, although few epidemiological data are available to address this point (see WARNINGS). The choice of progestin, its dose, and its regimen may be important in minimizing these adverse effects, but these issues will require further study before they are clarified.
-
Cardiovascular risk. The effects of estrogen replacement on the risk of cardiovascular disease have not been adequately studied. However, data from the Heart and Estrogen/Progestin Replacement Study (HERS), a controlled clinical trial of secondary prevention of 2,763 post-menopausal women with documented heart disease, demonstrated no benefit. During an average follow-up of 4.1 years, treatment with oral conjugated estrogen plus medroxyprogesterone acetate did not reduce the overall rate of coronary heart disease (CHD) events in post-menopausal women with established coronary disease. There were more CHD events in the hormone treated group than in the placebo group in year 1, but fewer events in years 3 through 5.
-
Physical examination. A complete medical and family history should be taken prior to the initiation of any estrogen therapy. The pretreatment and periodic physical examinations should include special reference to blood pressure, breasts, abdomen, and pelvic organs, and should include a Papanicolaou smear. As a general rule, estrogen should not be prescribed for longer than one year without reexamining the patient.
-
Hypercoagulability. Some studies have shown that women taking estrogen replacement therapy have hypercoagulability, primarily related to decreased antithrombin activity. This effect appears dose- and duration-dependent and is less pronounced than that associated with oral contraceptive use. Also, postmenopausal women tend to have increased coagulation parameters at baseline compared to premenopausal women. There is some suggestion that low dose postmenopausal mestranol may increase the risk of thromboembolism, although the majority of studies (of primarily conjugated estrogens users) report no such increase. There is insufficient information on hypercoagulability in women who have had previous thromboembolic disease.
-
Familial hyperlipoproteinemia. Estrogen therapy may be associated with massive elevations of plasma triglycerides leading to pancreatitis and other complications in patients with familial defects of lipoprotein metabolism.
-
Fluid retention. Because estrogens may cause some degree of fluid retention, conditions which might be exacerbated by this factor, such as asthma, epilepsy, migraine, and cardiac or renal dysfunction, require careful observation.
-
Uterine bleeding and mastodynia. Certain patients may develop undesirable manifestations of estrogenic stimulation, such as abnormal uterine bleeding and mastodynia.
-
Impaired liver function. Estrogens may be poorly metabolized in patients with impaired liver function and should be administered with caution.
B. Information for the Patient.
See text of Patient Package Insert.
C. Laboratory Tests.
Estrogen administration should generally be guided by clinical response at the smallest dose, rather than laboratory monitoring, for relief of symptoms for those indications in which symptoms are observable.
D. Drug/Laboratory Test Interactions.
-
Accelerated prothrombin time, partial thromboplastin time, and platelet aggregation time; increased platelet count; increased factors II, VII antigen, VIII antigen, VIII coagulant activity, IX, X, XII, VII-X complex, II-VII-X complex, and beta-thromboglobulin; decreased levels of anti-factor Xa and antithrombin III, decreased antithrombin III activity; increased levels of fibrinogen and fibrinogen activity; increased plasminogen antigen and activity.
-
Increased thyroid-binding globulin (TBG) leading to increased circulating total thyroid hormone, as measured by protein-bound iodine (PBI), T4 levels (by column or by radioimmunoassay) or T3 levels by radioimmunoassay. T3 resin uptake is decreased, reflecting the elevated TBG. Free T4 and free T3 concentrations are unaltered.
-
Other binding proteins may be elevated in serum, i.e., corticosteroid binding globulin (CBG), sex hormone-binding globulin (SHBG), leading to increased circulating corticosteroids and sex steroids, respectively. Free or biologically active hormone concentrations are unchanged. Other plasma proteins may be increased (angiotensinogen/renin substrate, alpha-1-antitrypsin, ceruloplasmin).
-
Increased plasma HDL and HDL-2 subfraction concentrations, reduced LDL cholesterol concentration, increased triglycerides levels.
-
Impaired glucose tolerance.
-
Reduced response to metyrapone test. 7
-
Reduced serum folate concentration.
E. Carcinogenesis, Mutagenesis, and Impairment of Fertility.
Long term continuous administration of natural and synthetic estrogens in certain animal species increases the frequency of carcinomas of the breast, uterus, cervix, vagina, testis, and liver. See CONTRAINDICATIONS and WARNINGS.
F. Pregnancy Category X.
Estrogens should not be used during pregnancy. See CONTRAINDICATIONS and Boxed WARNINGS.
G. Nursing Mothers.
As a general principle, the administration of any drug to nursing mothers should be done only when clearly necessary since many drugs are excreted in human milk. In addition, estrogen administration to nursing mothers has been shown to decrease the quantity and quality of the milk.
H. Pediatric Use.
Safety and effectiveness in pediatric patients have not been established. Large and repeated doses of estrogen over an extended period of time may accelerate epiphyseal closure. Therefore, periodic monitoring of bone maturation and effects on epiphyseal centers is recommended in patients in whom bone growth is not complete.
Adverse Reactions
The following additional adverse reactions have been reported with estrogen therapy (see WARNINGS regarding induction of neoplasia, adverse effects on the fetus, increased incidence of gallbladder disease, cardiovascular disease, elevated blood pressure, and hypercalcemia).
-
Genitourinary system.
Changes in vaginal bleeding pattern and abnormal withdrawal bleeding or flow; breakthrough bleeding, spotting.
Increase in size of uterine leiomyomata.
Vaginal candidiasis.
Change in amount of cervical secretion.
-
Breasts.
Tenderness, enlargement.
-
Gastrointestinal.
Nausea, vomiting.
Abdominal cramps, bloating.
Cholestatic jaundice.
Increased incidence of gallbladder disease.
-
Skin.
Chloasma or melasma that may persist when drug is discontinued.
Erythema multiforme.
Erythema nodosum.
Hemorrhagic eruption.
Loss of scalp hair.
Hirsutism.
-
Eyes.
Steepening of corneal curvature.
Intolerance to contact lenses.
-
Central Nervous System.
Headache, migraine, dizziness.
Mental depression.
Chorea.
-
Miscellaneous.
Increase or decrease in weight.
Reduced carbohydrate tolerance.
Aggravation of porphyria.
Edema.
Changes in libido.
Overdosage
Serious ill effects have not been reported following acute ingestion of large doses of estrogen-containing oral contraceptives by young children. Overdosage of estrogen may cause nausea and vomiting, and withdrawal bleeding may occur in females.
Dosage and Administration
Care should be taken to inject deeply into the upper, outer quadrant of the gluteal muscle following the usual precautions for intramuscular administration. By virtue of the low viscosity of the vehicles, the various preparations of DELESTROGEN (estradiol valerate injection, USP) may be administered with a small gauge needle. Since the 40 mg potency provides a high concentration in a small volume, particular care should be observed to administer the full dose.
DELESTROGEN should be visually inspected for particulate matter and color prior to administration; the solution is clear, colorless to pale yellow. Storage at low temperatures may result in the separation of some crystalline material which redissolves readily on warming.
Note: A dry needle and syringe should be used. Use of a wet needle or syringe may cause the solution to become cloudy; however, this does not affect the potency of the material.
-
For treatment of moderate to severe vasomotor symptoms, vulval and vaginal atrophy associated with the menopause, the lowest dose and regimen that will control symptoms should be chosen and medication should be discontinued as promptly as possible.
Attempts to discontinue or taper medication should be made at 3-month to 6-month intervals.
The usual dosage is 10 to 20 mg DELESTROGEN every four weeks.
-
For treatment of female hypoestrogenism due to hypogonadism, castration, or primary ovarian failure.
The usual dosage is 10 to 20 mg DELESTROGEN every four weeks.
-
For treatment of advanced androgen-dependent carcinoma of the prostate, for palliation only.
The usual dosage is 30 mg or more administered every one or two weeks.
Treated patients with an intact uterus should be monitored closely for signs of endometrial cancer, and appropriate diagnostic measures should be taken to rule out malignancy in the event of persistent or recurring abnormal vaginal bleeding.
See PRECAUTIONS A.1 concerning addition of a progestin.
How Supplied
DELESTROGEN® (estradiol valerate injection, USP)
Multiple Dose Vials
10 mg/mL (5 mL): NDC 61570-180-01
20 mg/mL (5 mL): NDC 61570-181-01
40 mg/mL (5 mL): NDC 61570-182-01
Storage
Store at room temperature.
Information for the Patient About
DELESTROGEN®
(estradiol valerate injection, USP)
INTRODUCTION
This leaflet describes when and how to use estrogens, and the risks and benefits of estrogen treatment.
Estrogens have important benefits but also some risks. You must decide, with your doctor, whether the risks to you of estrogen use are acceptable because of their benefits. If you use estrogens, check with your doctor to be sure you are using the lowest possible dose that works, and that you don’t use them longer than necessary. How long you need to use estrogens will depend on the reason for use.
WARNINGS
-
ESTROGENS INCREASE THE RISK OF CANCER OF THE UTERUS IN WOMEN WHO HAVE HAD THEIR MENOPAUSE (“CHANGE OF LIFE”).
If you use any estrogen-containing drug, it is important to visit your doctor regularly and report any unusual vaginal bleeding right away. Vaginal bleeding after menopause may be a warning sign of uterine cancer. Your doctor should evaluate any unusual vaginal bleeding to find out the cause.
-
ESTROGENS SHOULD NOT BE USED DURING PREGNANCY.
Estrogens do not prevent miscarriage (spontaneous abortion) and are not needed in the days following childbirth. If you take estrogens during pregnancy, your unborn child has a greater than usual chance of having birth defects. The risk of developing these defects is small, but clearly larger than the risk in children whose mothers did not take estrogens during pregnancy. These birth defects may affect the baby’s urinary system and sex organs. Daughters born to mothers who took DES (an estrogen drug) have a higher than usual chance of developing cancer of the vagina or cervix when they become teenagers or young adults. Sons may have a higher than usual chance of developing cancer of the testicles when they become teenagers or young adults.
USES OF ESTROGEN
(Not every estrogen drug is approved for every use listed in this section. If you want to know which of these possible uses are approved for the medicine prescribed for you, ask your doctor or pharmacist to show you the professional labeling. You can also look up the specific estrogen product in a book called the “Physicians’ Desk Reference”, which is available in many book stores and public libraries. Generic drugs carry virtually the same labeling information as their brand name versions.)
-
To reduce moderate or severe menopausal symptoms.
Estrogens are hormones made by the ovaries of normal women. Between ages 45 and 55, the ovaries normally stop making estrogens. This leads to a drop in body estrogen levels which causes the “change of life’’ or menopause (the end of monthly menstrual periods). If both ovaries are removed during an operation before natural menopause takes place, the sudden drop in estrogen levels causes “surgical menopause”.
When the estrogen levels begin dropping, some women develop very uncomfortable symptoms, such as feelings of warmth in the face, neck, and chest, or sudden intense episodes of heat and sweating (“hot flashes” or “hot flushes”). Using estrogen drugs can help the body adjust to lower estrogen levels and reduce these symptoms. Most women have only mild menopausal symptoms or none at all and do not need to use estrogen drugs for these symptoms. Others may need to take estrogens for a few months while their bodies adjust to lower estrogen levels. The majority of women do not need estrogen replacement for longer than six months for these symptoms.
-
To treat vulval and vaginal atrophy (itching, burning, dryness in or around the vagina, difficulty or burning on urination) associated with menopause.
-
To treat certain conditions in which a young woman’s ovaries do not produce enough estrogen naturally.
-
To treat certain types of abnormal vaginal bleeding due to hormonal imbalance when your doctor has found no serious cause of the bleeding.
-
To treat certain cancers in special situations, in men and women.
-
To prevent thinning of bones.
Osteoporosis is a thinning of the bones that makes them weaker and allows them to break more easily. The bones of the spine, wrists and hips break most often in osteoporosis. Both men and women start to lose bone mass after about age 40, but women lose bone mass faster after the menopause. Using estrogens after the menopause slows down bone thinning and may prevent bones from breaking. Lifelong adequate calcium intake, either in the diet (such as dairy products) or by calcium supplements (to reach a total daily intake of 1000 milligrams per day before menopause or 1500 milligrams per day after menopause), may help to prevent osteoporosis. Regular weight-bearing exercise (like walking and running for an hour, two or three times a week) may also help to prevent osteoporosis. Before you change your calcium intake or exercise habits, it is important to discuss these lifestyle changes with your doctor to find out if they are safe for you.
Since estrogen use has some risks, only women who are likely to develop osteoporosis should use estrogens for prevention. Women who are likely to develop osteoporosis often have the following characteristics: white or Asian race, slim, cigarette smokers, and a family history of osteoporosis in a mother, sister, or aunt. Women who have relatively early menopause, often because their ovaries were removed during an operation (“surgical menopause”), are more likely to develop osteoporosis than women whose menopause happens at the average age.
WHO SHOULD NOT USE ESTROGENS
Estrogens should not be used:
-
During pregnancy (see Boxed WARNINGS).
If you think you may be pregnant, do not use any form of estrogen-containing drug. Using estrogens while you are pregnant may cause your unborn child to have birth defects. Estrogens do not prevent miscarriage.
-
If you have unusual vaginal bleeding which has not been evaluated by your doctor (see Boxed WARNINGS).
Unusual vaginal bleeding can be a warning sign of cancer of the uterus, especially if it happens after menopause. Your doctor must find out the cause of the bleeding so that he or she can recommend the proper treatment. Taking estrogens without visiting your doctor can cause you serious harm if your vaginal bleeding is caused by cancer of the uterus.
-
If you have had cancer.
Since estrogens increase the risk of certain types of cancer, you should not use estrogens if you have ever had cancer of the breast or uterus, unless your doctor recommends that the drug may help in the cancer treatment. (For certain patients with breast or prostate cancer, estrogens may help.)
-
If you have any circulation problems.
Estrogen drugs should not be used except in unusually special situations in which your doctor judges that you need estrogen therapy so much that the risks are acceptable. Men and women with abnormal blood clotting conditions should avoid estrogen use (see DANGERS OF ESTROGENS).
-
When they do not work.
During menopause, some women develop nervous symptoms or depression. Estrogens do not relieve these symptoms. You may have heard that taking estrogens for years after menopause will keep your skin soft and supple and keep you feeling young. There is no evidence for these claims and such long-term estrogen use may have serious risks.
-
After childbirth or when breastfeeding a baby.
Estrogens should not be used to try to stop the breasts from filling with milk after a baby is born. Such treatment may increase the risk of developing blood clots (see DANGERS OF ESTROGENS).
If you are breastfeeding, you should avoid using any drugs because many drugs pass through to the baby in the milk. While nursing a baby, you should take drugs only on the advice of your health care provider.
DANGERS OF ESTROGENS
-
Cancer of the uterus. Your risk of developing cancer of the uterus gets higher the longer you use estrogens and the larger doses you use. One study showed that after women stop taking estrogens, this higher cancer risk quickly returns to the usual level of risk (as if you had never used estrogen therapy). Three other studies showed that the cancer risk stayed high for 8 to more than 15 years after stopping estrogen treatment. Because of this risk, IT IS IMPORTANT TO TAKE THE LOWEST DOSE THAT WORKS AND TO TAKE IT ONLY AS LONG AS YOU NEED IT.
Using progestin therapy together with estrogen therapy may reduce the higher risk of uterine cancer related to estrogen use (but see OTHER INFORMATION).
If you have had your uterus removed (total hysterectomy), there is no danger of developing cancer of the uterus.
-
Cancer of the breast.
Studies examining the risk of breast cancer among women using estrogen alone and combined estrogen/progestin therapy have suggested that there may be a mildly increased risk of breast cancer in women taking the combined therapy.
If you do not have your uterus, there is no need for combined estrogen/progestin therapy since estrogen alone therapy is sufficient and may pose less risk for breast cancer.
If you do have your uterus, you should discuss the benefits and risks of combined estrogen/progestin therapy with your health care provider. Regular breast exams by a health professional and monthly self-exams are recommended for all women. Mammography may also be recommended depending on your age and risk factors.
-
Gallbladder disease.
Women who use estrogens after menopause are more likely to develop gallbladder disease needing surgery than women who do not use estrogens.
-
Abnormal blood clotting.
Taking estrogens may cause changes in your blood clotting system. These changes allow the blood to clot more easily, possibly allowing clots to form in your bloodstream. If blood clots do form in your bloodstream, they can cut off the blood supply to vital organs, causing serious problems. These problems may include a stroke (by cutting off blood to the brain), a heart attack (by cutting off blood to the heart), a pulmonary embolus (by cutting off blood to the lungs), or other problems. Any of these conditions may cause death or serious long term disability. However, most studies of low dose estrogen usage by women do not show an increased risk of these complications.
SIDE EFFECTS
In addition to the risks listed above, the following side effects have been reported with estrogen use.
Nausea and vomiting.
Breast tenderness or enlargement.
Enlargement of benign tumors (“fibroids”) of the uterus.
Retention of excess fluid. This may make some conditions worsen, such as asthma, epilepsy, migraine, heart disease, or kidney disease.
A spotty darkening of the skin, particularly on the face.
REDUCING RISK OF ESTROGEN USE
If you use estrogens, you can reduce your risks by doing these things:
-
See your doctor regularly.
While you are using estrogens, it is important to visit your doctor at least once a year for a check-up. If you develop vaginal bleeding while taking estrogens, you may need further evaluation. If members of your family have had breast cancer or if you have ever had breast lumps or an abnormal mammogram (breast x-ray), you may need to have more frequent breast examinations.
-
Reassess your need for estrogens.
You and your doctor should reevaluate whether or not you still need estrogens at least every six months.
-
Be alert for signs of trouble.
If any of these warning signals (or any other unusual symptoms) happen while you are using estrogens, call your doctor immediately:
Abnormal bleeding from the vagina (possible uterine cancer)
Pains in the calves or chest, sudden shortness of breath, or coughing blood (possible clot in the legs, heart, or lungs)
Severe headache or vomiting, dizziness, faintness, changes in vision or speech, weakness or numbness of an arm or leg (possible clot in the brain or eye)
Breast lumps (possible breast cancer; ask your doctor or health professional to show you how toexamine your breasts monthly)
Yellowing of the skin or eyes (possible liver problem)
Pain, swelling, or tenderness in the abdomen (possible gallbladder problem)
OTHER INFORMATION
-
Estrogens increase the risk of developing a condition (endometrial hyperplasia) that may lead to cancer of the lining of the uterus. Taking progestins, another hormone drug, with estrogens lowers the risk of developing this condition. Therefore, if your uterus has not been removed, your doctor may prescribe a progestin for you to take together with the estrogen.
You should know, however, that taking estrogens with progestins may have additional risks. These include: (a) unhealthy effects on blood fats (especially the lowering of HDL blood cholesterol, the “good” blood fat which protects against heart disease); (b) unhealthy effects on blood sugar (which might make a diabetic condition worse); and (c) a possible further increase in breast cancer risk which may be associated with long-term estrogen use.
Some research has shown that estrogens taken without progestins may protect women against developing heart disease. However, this is not certain. The protection shown may have been caused by the characteristics of the estrogentreated women, and not by the estrogen treatment itself. In general, treated women were slimmer, more physically active, and were less likely to have diabetes than the untreated women. These characteristics are known to be associated with a reduced risk for heart disease.
You are cautioned to discuss very carefully with your doctor or health care provider all the possible risks and benefits of long-term estrogen and progestin treatment as they affect you personally.
-
Your doctor has prescribed this drug for you and you alone. Do not give the drug to anyone else.
-
If you will be taking calcium supplements as part of the treatment to help prevent osteoporosis, check with your doctor about how much to take.
-
Keep this and all drugs out of the reach of children. In case of overdose, call your doctor, hospital or poison control center immediately.
-
This leaflet provides a summary of the most important information about estrogens. If you want more information, ask your doctor or pharmacist to show you the professional labeling. The professional labeling is also published in a book called the “Physicians’ Desk Reference,” which is available in book stores and public libraries. Generic drugs carry virtually the same labeling information as their brand name versions.
Distributed by: Monarch Pharmaceuticals, Inc., Bristol, TN 37620
Manufactured by: Bristol-Myers Squibb Company, Princeton, NJ 08543 USA
| Delestrogen (estradiol valerate) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Delestrogen (estradiol valerate) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Delestrogen (estradiol valerate) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Revised: 10/2006