evoxac (cevimeline hydrochloride) capsule
[Daiichi Pharmaceutical Corporation]
DESCRIPTION
Cevimeline is cis-2'-methylspiro {1-azabicyclo [2.2.2] octane-3, 5'–[1,3] oxathiolane} hydrochloride, hydrate (2:1). Its
empirical formula is C10H17NOS.HCI.1/2H20, and its structural formula is: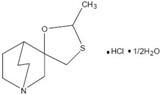
Cevimeline has a molecular weight of 244.79. It is a white to off white crystalline powder with a melting point range of 201 to 203°C. It is freely soluble in alcohol and chloroform, very soluble in water, and virtually insoluble in ether. The pH of a 1% solution ranges from 4.6 to 5.6. Inactive ingredients include lactose monohydrate, hydroxypropyl cellulose, and magnesium stearate.
CLINICAL PHARMACOLOGY
Pharmacodynamics
Cevimeline is a cholinergic agonist which binds to muscarinic receptors. Muscarinic agonists in sufficient dosage can increase secretion of exocrine glands, such as salivary and sweat glands and increase tone of the smooth muscle in the gastrointestinal and urinary tracts.
Pharmacokinetics
Absorption: After administration of a single 30 mg capsule, cevimeline was rapidly absorbed with a mean time to peak concentration of 1.5 to 2 hours. No accumulation of active drug or its metabolites was observed following multiple dose administration. When administered with food, there is a decrease in the rate of absorption, with a fasting TMAX of 1.53 hours and a TMAX of 2.86 hours after a meal; the peak concentration is reduced by 17.3%. Single oral doses across the clinical dose range are dose proportional.
Distribution: Cevimeline has a volume of distribution of approximately 6L/kg and is <20% bound to human plasma proteins. This suggests that cevimeline is extensively bound to tissues; however, the specific binding sites are unknown.
Metabolism: Isozymes CYP2D6 and CYP3A3/4 are responsible for the metabolism of cevimeline. After 24 hours, 86.7% of the dose was recovered (16.0% unchanged, 44.5% as cis and trans-sulfoxide, 22.3% of the dose as glucuronic acid conjugate and 4% of the dose as N-oxide of cevimeline). Approximately 8% of the trans-sulfoxide metabolite is then converted into the corresponding glucuronic acid conjugate and eliminated. Cevimeline did not inhibit cytochrome P450 isozymes 1A2, 2A6, 2C9, 2C19, 2D6, 2E1, and 3A4.
Excretion: The mean half-life of cevimeline is 5+/-1 hours. After 24 hours, 84% of a 30 mg dose of cevimeline was excreted in urine. After seven days, 97% of the dose was recovered in the urine and 0.5% was recovered in the feces.
Special Populations: The effects of renal impairment, hepatic impairment, or ethnicity on the pharmacokinetics of cevimeline have not been investigated.
Clinical Studies
Cevimeline has been shown to improve the symptoms of dry mouth in patients with Sjögren's Syndrome.
A 6-week, randomized, double blind, placebo-controlled study was conducted in 75 patients (10 men, 65 women) with a mean age of 53.6 years (range 33-75). The racial distribution was Caucasian 92%, Black 1% and other 7%. The effects of cevimeline at 30 mg tid (90 mg/day) and 60 mg tid (180 mg/day) were compared to those of placebo. Patients were evaluated by a measure called global improvement, which is defined as a response of “better” to the question, “Please rate the overall condition of your dry mouth now compared with how you felt before starting treatment in this study.” Patients also had the option of selecting “worse” or “no change” as answers. Seventy-six percent of the patients in the 30 mg tid group reported a global improvement in their dry mouth symptoms compared to 35% of the patients in the placebo group. This difference was statistically significant at p=0.0043. There was no evidence that patients in the 60 mg tid group had better global evaluation scores than the patients in the 30 mg tid group.
A 12-week, randomized, double-blind, placebo-controlled study was conducted in 197 patients (10 men, 187 women) with a mean age of 54.5 years (range 23-74). The racial distribution was Caucasian 91.4%, Black 3%, and other 5.6%. The effects of cevimeline at 15 mg tid (45 mg/day) and 30 mg tid (90 mg/day) were compared to those of placebo. Statistically significant global improvement in the symptoms of dry mouth (p=0.0004) was seen for the 30 mg tid group compared to placebo, but not for the 15 mg group compared to placebo. Salivary flow showed statistically significant increases at both doses of cevimeline during the study compared to placebo.
A second 12-week, randomized, double-blind, placebo-controlled study was conducted in 212 patients (11 men, 201 women) with a mean age of 55.3 years (range 24-75). The racial distribution was Caucasian 88.7%, Black 1.9% and other 9.4%. The effects of cevimeline at 15 mg tid (45 mg/day) and 30 mg tid (90 mg/day) were compared to those of placebo. No statistically significant differences were noted in the patient global evaluations. However, there was a higher placebo response rate in this study compared to the aforementioned studies. The 30 mg tid group showed a statistically significant increase in salivary flow from pre-dose to post-dose compared to placebo (p=0.0017).
INDICATIONS AND USAGE
Cevimeline is indicated for the treatment of symptoms of dry mouth in patients with Sjögren's Syndrome.
CONTRAINDICATIONS
Cevimeline is contraindicated in patients with uncontrolled asthma, known hypersensitivity to cevimeline, and when miosis is undesirable, e.g., in acute iritis and in narrow-angle (angle-closure) glaucoma.
WARNINGS
Cardiovascular Disease:
Cevimeline can potentially alter cardiac conduction and/or heart rate. Patients with significant cardiovascular disease may potentially be unable to compensate for transient changes in hemodynamics or rhythm induced by EVOXAC®. EVOXAC® should be used with caution and under close medical supervision in patients with a history of cardiovascular disease evidenced by angina pectoris or myocardial infarction.
Pulmonary Disease:
Cevimeline can potentially increase airway resistance, bronchial smooth muscle tone, and bronchial secretions. Cevimeline should be administered with caution and with close medical supervision to patients with controlled asthma, chronic bronchitis, or chronic obstructive pulmonary disease.
Ocular:
Ophthalmic formulations of muscarinic agonists have been reported to cause visual blurring which may result in decreased visual acuity, especially at night and in patients with central lens changes, and to cause impairment of depth perception. Caution should be advised while driving at night or performing hazardous activities in reduced lighting.
PRECAUTIONS
General:
Cevimeline toxicity is characterized by an exaggeration of its parasympathomimetic effects. These may include: headache, visual disturbance, lacrimation, sweating, respiratory distress, gastrointestinal spasm, nausea, vomiting, diarrhea, atrioventricular block, tachycardia, bradycardia, hypotension, hypertension, shock, mental confusion, cardiac arrhythmia, and tremors.
Cevimeline should be administered with caution to patients with a history of nephrolithiasis or cholelithiasis. Contractions of the gallbladder or biliary smooth muscle could precipitate complications such as cholecystitis, cholangitis and biliary obstruction. An increase in the ureteral smooth muscle tone could theoretically precipitate renal colic or ureteral reflux in patients with nephrolithiasis.
Information for Patients
Patients should be informed that cevimeline may cause visual disturbances, especially at night, that could impair their ability to drive safely.
If a patient sweats excessively while taking cevimeline, dehydration may develop. The patient should drink extra water and consult a health care provider.
Drug Interactions:
Cevimeline should be administered with caution to patients taking beta adrenergic antagonists, because of the possibility of conduction disturbances. Drugs with parasympathomimetic effects administered concurrently with cevimeline can be expected to have additive effects. Cevimeline might interfere with desirable antimuscarinic effects of drugs used concomitantly.
Drugs which inhibit CYP2D6 and CYP3A3/4 also inhibit the metabolism of cevimeline. Cevimeline should be used with caution in individuals known or suspected to be deficient in CYP2D6 activity, based on previous experience, as they may be at a higher risk of adverse events. In an in vitro study, cytochrome P450 isozymes 1A2, 2A6, 2C9, 2C19, 2D6, 2E1, and 3A4 were not inhibited by exposure to cevimeline.
Carcinogenesis, Mutagenesis and Impairment of Fertility:
Lifetime carcinogenicity studies were conducted in CD-1 mice and F-344 rats. A statistically significant increase in the incidence of adenocarcinomas of the uterus was observed in female rats that received cevimeline at a dosage of 100 mg/kg/day (approximately 8 times the maximum human exposure based on comparison of AUC data). No other significant differences in tumor incidence were observed in either mice or rats.
Cevimeline exhibited no evidence of mutagenicity or clastogenicity in a battery of assays that included an Ames test, an in vitro chromosomal aberration study in mammalian cells, a mouse lymphoma study in L5178Y cells, or a micronucleus assay conducted in vivo in ICR mice.
Cevimeline did not adversely affect the reproductive performance of fertility of male Sprague-Dawley rats when administered for 63 days prior to mating and throughout the period of mating at dosages up to 45 mg/kg/day (approximately 5 times the maximum recommended dose for a 60 kg human following normalization of the data on the basis of body surface area estimates). Females that were treated with cevimeline at dosages up to 45 mg/kg/day from14 days prior to mating through day seven of gestation exhibited a statistically significantly smaller number of implantations than did control animals.
Pregnancy
Pregnancy Category C.
Cevimeline was associated with a reduction in the mean number of implantations when given to pregnant Sprague-Dawley rats from 14 days prior to mating through day seven of gestation at a dosage of 45 mg/kg/day (approximately 5 times the maximum recommended dose for a 60 kg human when compared on the basis of body surface area estimates). This effect may have been secondary to maternal toxicity. There are no adequate and well-controlled studies in pregnant women. Cevimeline should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers:
It is not known whether this drug is secreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from EVOXAC®, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use:
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use:
Although clinical studies of cevimeline included subjects over the age of 65, the numbers were not sufficient to determine whether they respond differently from younger subjects. Special care should be exercised when cevimeline treatment is initiated in an elderly patient, considering the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy in the elderly.
ADVERSE REACTIONS
Cevimeline was administered to 1777 patients during clinical trials worldwide, including Sjögren's patients and patients with other conditions. In placebo-controlled Sjögren's studies in the U.S., 320 patients received cevimeline doses ranging from 15 mg tid to 60 mg tid, of whom 93% were women and 7% were men. Demographic distribution was 90% Caucasian, 5% Hispanic, 3% Black and 2% of other origin. In these studies, 14.6% of patients discontinued treatment with cevimeline due to adverse events.
The following adverse events associated with muscarinic agonism were observed in the clinical trials of cevimeline in Sjögren's syndrome patients:
| Adverse Event | Cevimeline 30 mg |
Placebo |
|---|---|---|
| (tid) n*=533 |
(tid) n=164 |
|
|
*n is the total number of patients exposed to the dose at any time during the study. |
||
| Excessive Sweating | 18.7% | 2.4% |
| Nausea | 13.8% | 7.9% |
| Rhinitis | 11.2% | 5.4% |
| Diarrhea | 10.3% | 10.3% |
| Excessive Salivation | 2.2% | 0.6% |
| Urinary Frequency | 0.9% | 1.8% |
| Asthenia | 0.5% | 0.0% |
| Flushing | 0.3% | 0.6% |
| Polyuria | 0.1% | 0.6% |
In addition, the following adverse events (≥3% incidence) were reported in the Sjögren's clinical trials:
| Adverse Event | Cevimeline 30 mg |
Placebo |
|---|---|---|
| (tid) n*=533 |
(tid) n=164 |
|
|
*n is the total number of patients exposed to the dose at any time during the study. |
||
| Headache | 14.4% | 20.1% |
| Sinusitis | 12.3% | 10.9% |
| Upper Respiratory Tract Infection | 11.4% | 9.1% |
| Dyspepsia | 7.8% | 8.5% |
| Abdominal Pain | 7.6% | 6.7% |
| Urinary Tract Infection | 6.1% | 3.0% |
| Coughing | 6.1% | 3.0% |
| Pharyngitis | 5.2% | 5.4% |
| Vomiting | 4.6% | 2.4% |
| Injury | 4.5% | 2.4% |
| Back Pain | 4.5% | 4.2% |
| Rash | 4.3% | 6.0% |
| Conjunctivitis | 4.3% | 3.6% |
| Dizziness | 4.1% | 7.3% |
| Bronchitis | 4.1% | 1.2% |
| Arthralgia | 3.7% | 1.8% |
| Surgical Intervention | 3.3% | 3.0% |
| Fatigue | 3.3% | 1.2% |
| Pain | 3.3% | 3.0% |
| Skeletal Pain | 2.8% | 1.8% |
| Insomnia | 2.4% | 1.2% |
| Hot Flushes | 2.4% | 0.0% |
| Rigors | 1.3% | 1.2% |
| Anxiety | 1.3% | 1.2% |
The following events were reported in Sjögren's patients at incidences of <3% and ≥1%: constipation, tremor, abnormal vision, hypertonia, peripheral edema, chest pain, myalgia, fever, anorexia, eye pain, earache, dry mouth, vertigo, salivary gland pain, pruritus, influenza-like symptoms, eye infection, post-operative pain, vaginitis, skin disorder, depression, hiccup, hyporeflexia, infection, fungal infection, sialoadenitis, otitis media, erythematous rash, pneumonia, edema, salivary gland enlargement, allergy, gastroesophageal reflux, eye abnormality, migraine, tooth disorder, epistaxis, flatulence, toothache, ulcerative stomatitis, anemia, hypoesthesia, cystitis, leg cramps, abscess, eructation, moniliasis, palpitation, increased amylase, xerophthalmia, allergic reaction.
The following events were reported rarely in treated Sjögren's patients (<1%): Causal relation is unknown:
Body as a Whole Disorders: aggravated allergy, precordial chest pain, abnormal crying, hematoma, leg pain, edema, periorbital edema, activated pain trauma, pallor, changed sensation temperature, weight decrease, weight increase, choking, mouth edema, syncope, malaise, face edema, substernal chest pain
Cardiovascular Disorders: abnormal ECG, heart disorder, heart murmur, aggravated hypertension, hypotension, arrhythmia, extrasystoles, t wave inversion, tachycardia, supraventricular tachycardia, angina pectoris, myocardial infarction, pericarditis, pulmonary embolism, peripheral ischemia, superficial phlebitis, purpura, deep thrombophlebitis, vascular disorder, vasculitis, hypertension
Digestive Disorders: appendicitis, increased appetite, ulcerative colitis, diverticulitis, duodenitis, dysphagia, enterocolitis, gastric ulcer, gastritis, gastroenteritis, gastrointestinal hemorrhage, gingivitis, glossitis, rectum hemorrhage, hemorrhoids, ileus, irritable bowel syndrome, melena, mucositis, esophageal stricture, esophagitis, oral hemorrhage, peptic ulcer, periodontal destruction, rectal disorder, stomatitis, tenesmus, tongue discoloration, tongue disorder, geographic tongue, tongue ulceration, dental caries
Endocrine Disorders: increased glucocorticoids, goiter, hypothyroidism
Hematologic Disorders: thrombocytopenic purpura, thrombocythemia, thrombocytopenia, hypochromic anemia, eosinophilia, granulocytopenia, leucopenia, leukocytosis, cervical lymphadenopathy, lymphadenopathy
Liver and Biliary System Disorders: cholelithiasis, increased gamma-glutamyl transferase, increased hepatic enzymes, abnormal hepatic function, viral hepatitis, increased serum glutamate oxaloacetic transaminase (SGOT) (also called AST-aspartate aminotransferase), increased serum glutamate pyruvate transaminase (SGPT) (also called ALT-alanine aminotransferase)
Metabolic and Nutritional Disorders: dehydration, diabetes mellitus, hypercalcemia, hypercholesterolemia, hyperglycemia, hyperlipemia, hypertriglyceridemia, hyperuricemia, hypoglycemia, hypokalemia, hyponatremia, thirst
Musculoskeletal Disorders: arthritis, aggravated arthritis, arthropathy, femoral head avascular necrosis, bone disorder, bursitis, costochondritis, plantar fasciitis, muscle weakness, osteomyelitis, osteoporosis, synovitis, tendonitis, tenosynovitis
Neoplasms: basal cell carcinoma, squamous carcinoma
Nervous Disorders: carpal tunnel syndrome, coma, abnormal coordination, dysesthesia, dyskinesia, dysphonia, aggravated multiple sclerosis, involuntary muscle contractions, neuralgia, neuropathy, paresthesia, speech disorder, agitation, confusion, depersonalization, aggravated depression, abnormal dreaming, emotional lability, manic reaction, paroniria, somnolence, abnormal thinking, hyperkinesia, hallucination
Miscellaneous Disorders: fall, food poisoning, heat stroke, joint dislocation, post-operative hemorrhage
Resistance Mechanism Disorders: cellulites, herpes simplex, herpes zoster, bacterial infection, viral infection, genital moniliasis, sepsis
Respiratory Disorders: asthma, bronchospasm, chronic obstructive airway disease, dyspnea, hemoptysis, laryngitis, nasal ulcer, pleural effusion, pleurisy, pulmonary congestion, pulmonary fibrosis, respiratory disorder
Rheumatologic Disorders: aggravated rheumatoid arthritis, lupus erythematosus rash, lupus erythematosus syndrome
Skin and Appendages Disorders: acne, alopecia, burn, dermatitis, contact dermatitis, lichenoid dermatitis, eczema, furunculosis, hyperkeratosis, lichen planus, nail discoloration, nail disorder, onychia, onychomycosis, paronychia, photosensitivity reaction, rosacea, scleroderma, seborrhea, skin discoloration, dry skin, skin exfoliation, skin hypertrophy, skin ulceration, urticaria, verruca, bullous eruption, cold clammy skin
Special Senses Disorders: deafness, decreased hearing, motion sickness, parosmia, taste perversion, blepharitis, cataract, corneal opacity, corneal ulceration, diplopia, glaucoma, anterior chamber eye hemorrhage, keratitis, keratoconjunctivitis, mydriasis, myopia, photopsia, retinal deposits, retinal disorder, scleritis, vitreous detachment, tinnitus
Urogenital Disorders: epididymitis, prostatic disorder, abnormal sexual function, amenorrhea, female breast neoplasm, malignant female breast neoplasm, female breast pain, positive cervical smear test, dysmenorrhea, endometrial disorder, intermenstrual bleeding, leukorrhea, menorrhagia, menstrual disorder, ovarian cyst, ovarian disorder, genital pruritus, uterine hemorrhage, vaginal hemorrhage, atrophic vaginitis, albuminuria, bladder discomfort, increased blood urea nitrogen, dysuria, hematuria, micturition disorder, nephrosis, nocturia, increased nonprotein nitrogen, pyelonephritis, renal calculus, abnormal renal function, renal pain, strangury, urethral disorder, abnormal urine, urinary incontinence, decreased urine flow, pyuria
In one subject with lupus erythematosus receiving concomitant multiple drug therapy, a highly elevated ALT level was noted after the fourth week of cevimeline therapy. In two other subjects receiving cevimeline in the clinical trials, very high AST levels were noted. The significance of these findings is unknown.
Additional adverse events (relationship unknown) which occurred in other clinical studies (patient population different from Sjögren's patients) are as follows:
cholinergic syndrome, blood pressure fluctuation, cardiomegaly, postural hypotension, aphasia, convulsions, abnormal gait, hyperesthesia, paralysis, abnormal sexual function, enlarged abdomen, change in bowel habits, gum hyperplasia, intestinal obstruction, bundle branch block, increased creatine phosphokinase, electrolyte abnormality, glycosuria, gout, hyperkalemia, hyperproteinemia, increased lactic dehydrogenase (LDH), increased alkaline phosphatase, failure to thrive, abnormal platelets, aggressive reaction, amnesia, apathy, delirium, delusion, dementia, illusion, impotence, neurosis, paranoid reaction, personality disorder, hyperhemoglobinemia, apnea, atelectasis, yawning, oliguria, urinary retention, distended vein, lymphocytosis
Post-marketing Adverse Events: cholecystitis
MANAGEMENT OF OVERDOSE
Management of the signs and symptoms of acute overdosage should be handled in a manner consistent with that indicated for other muscarinic agonists: general supportive measures should be instituted. If medically indicated, atropine, an anti-cholinergic agent, may be of value as an antidote for emergency use in patients who have had an overdose of cevimeline. If medically indicated, epinephrine may also be a value in the presence of severe cardiovascular depression or bronchoconstriction. It is not known if cevimeline is dialyzable.
DOSAGE AND ADMINISTRATION
The recommended dose of cevimeline hydrochloride is 30 mg taken three times a day. There is insufficient safety information to support doses greater than 30 mg tid. There is also insufficient evidence for additional efficacy of cevimeline hydrochloride at doses greater than 30 mg tid.
HOW SUPPLIED
EVOXAC® is available as white, hard gelatin capsules containing 30 mg of cevimeline hydrochloride. EVOXAC® capsules have a white opaque cap and a white opaque body. The capsules are imprinted with “EVOXAC” on the cap and “30 mg” on the body with a black bar above “30 mg”. It is supplied in child resistant bottles of:
100 capsules (NDC 63395-201-13)
Store at 25°C (77°F) excursion permitted to 15° -30°C (59° -86°F)
Rx Only
Distributed and Marketed by:
Daiichi Pharmaceutical Corporation
Montvale, NJ 07645
EVOXAC is a registered trademark of
Daiichi Pharmaceutical Co., Ltd.
PRT30 Revised 04/2005 Printed in U.S.A.
A patient's guide to managing dry-mouth symptoms of SjÖgren's syndrome with EVOXAC® (cevimeline HCI)
What is EVOXAC® (cevimeline HCI) and how can it help me?
EVOXAC (‘è vô zak) is a product available by prescription only that can help to relieve your dry-mouth symptoms due to Sjögren's syndrome.
EVOXAC acts on your salivary glands, helping them to produce more saliva. With more saliva, the dry feeling in your mouth will begin to lessen, which should make it easier to eat and speak without needing to sip water constantly. This may even help you to sleep better.¹
More than 680 patients with Sjögren's syndrome took part in medical studies of EVOXAC before it became available by prescription. The patients who took EVOXAC had an increase in their saliva flow compared with the patients who took a sugar pill—or placebo. Many of the patients who took EVOXAC also said their dry-mouth symptoms felt better.
How is EVOXAC different from other treatments for the dry-mouth symptoms of Sjögren's syndrome?
Many Sjögren's syndrome patients sip water contantly, suck on sugar-free lozenges or candy, chew gum, or use artificial saliva. These methods provide some immediate moisture, but they don't restore an adequate flow of saliva. EVOXAC is different—it stimulates the natural flow of saliva to help you to eat and speak.
Who shouldn't take EVOXAC?
You should not take EVOXAC if you have:
- Uncontrolled asthma
- Acute iritis
- Narrow-angle (angle-closure) glaucoma
- An allergy to EVOXAC
The safety and effectiveness of EVOXAC in patients less than 18 years of age have not been established.
What should you tell your health care provider before taking EVOXAC?
Before taking EVOXAC, you should tell your health care provider if you have:
- A heart condition
- Controlled asthma
- Chronic bronchitis
- Emphysema
- A history of kidney stones or gallstones
Also tell your health care provider if you have been taking any heart medication, especially “beta-blockers.” If you have any of these conditions, your health care provider will decide whether EVOXAC is right for you and if so, your health care provider should monitor you under close medical supervision while you are taking EVOXAC.
What are the most common side effects of EVOXAC?
Some patients taking EVOXAC may experience side effects including:
- Excessive sweating
- Headache
- Nausea
- Sinusitis
- Upper respiratory tract infection
- Runny nose (rhinitis)
- Diarrhea
Some of these side effects may be signs the drug is beginning to work. For example, if you find that you are sweating more after you start taking EVOXAC, it is because EVOXAC is causing your sweat glands to increase their activity.
If any of these side effects persist or become bothersome, or you experience another side effect that you think may be related to your therapy with EVOXAC, you should tell your health care provider as soon as possible. You and your health care provider will decide how to best manage side effects.
Additional safety information
If you sweat excessively while taking EVOXAC and don't drink enough fluid, you may become dehydrated. To prevent this, always maintain an adequate fluid intake. If you feel you are becoming dehydrated, increase your fluid intake and consult with your health care provider.
You should be careful while driving at night or performing hazardous activities in reduced lighting while taking EVOXAC.
Special care should be taken in elderly patients.
Do I need to tell my health care provider about other medications I am taking before taking EVOXAC?
Yes. Before taking EVOXAC, you should tell your health care provider if you are taking any heart medication, especially “beta-blockers.” You should also inform your health care provider and your pharmacist of all prescription and over-the-counter medications you are taking, to avoid any possible drug interactions.
How should I take EVOXAC?
Take EVOXAC exactly as directed by your health care provider. EVOXAC is usually taken as one capsule three times a day.
What should I do if I miss a dose?
Missing a dose may make your medication less effective. Do not take two doses at once to make up for the missed dose. If you are uncertain about when to take your next dose, contact your health care provider.
How long may it take for EVOXAC to start to work?
Each patient responds differently. Some patients respond soon after taking EVOXAC, others respond over time, and others do not respond after taking EVOXAC.¹ It is important to follow your health care provider's instructions. The samples you have received will only last a limited time. An adequate trial of therapy usually requires filling at least one prescription.
Please see other side for prescribing information about the risks and benefits of taking EVOXAC.
Additional resources for Sjögren's syndrome patients:
Sjögren's Syndrome Foundation
1-800-475-6473
www.sjogrens.org
National Sjögren's Syndrome Association
1-800-395-NSSA (6772)
www.sjogrenssyndrome.org
Arthritis Foundation
1-800-283-7800
www.arthritis.org
Sjögren's Syndrome Clinic
National Institute of Dental and Craniofacial Research
1-301-435-8528
wwwdir.nidcr.nih.gov/sjogrens/SjogrenMain.htm
Daiichi Pharmaceutical Corporation has no control over the content of these websites.
Evoxac®
(cevimeline HCL)30 mg Capsules
Proven Relief…
Proven Results
Daiichi Pharmaceutical Corporation
www.daiichius.com
Reference: 1. Data on file, Daiichi Pharmaceutical Corporation.
EVOXAC is a registered trademark of Daiichi Pharmaceutical Co., Ltd.
© 2005 Daiichi Pharmaceutical Corportation
EV-201-217B/R Printed in USA 04/05 PRT31
| EVOXAC (cevimeline hydrochloride) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Revised: 01/2006Daiichi Pharmaceutical Corporation