kenalog (triamcinolone acetonide) injection, suspension
[Bristol-Myers Squibb Company]
For Intra-articular, Intrabursal or Intradermal Use
NOT FOR INTRAVENOUS OR INTRAMUSCULAR USE
DESCRIPTION
Kenalog-10 Injection (Triamcinolone Acetonide Injectable Suspension, USP) provides triamcinolone acetonide, a synthetic corticosteroid with marked anti-inflammatory action, in a sterile aqueous suspension suitable for intradermal, intra-articular, and intrabursal injection and for injection into tendon sheaths. This preparation is NOT suitable for intravenous or intramuscular use. Each mL of the sterile aqueous suspension provides 10 mg triamcinolone acetonide, with sodium chloride for isotonicity, 0.9% (w/v) benzyl alcohol as a preservative, 0.75% carboxymethylcellulose sodium, and 0.04% polysorbate 80; sodium hydroxide or hydrochloric acid may have been added to adjust pH between 5.0 and 7.5. At the time of manufacture, the air in the container is replaced by nitrogen.
The chemical name for triamcinolone acetonide is 9-fluoro-11β, 16α, 17,21-tetrahydroxy-pregna-1,4-diene-3,20-dione cyclic 16,17-acetal with acetone; its structural formula is:
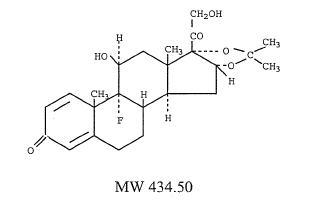
CLINICAL PHARMACOLOGY
Naturally occurring glucocorticoids (hydrocortisone), which also have salt-retaining properties, are used as replacement therapy in adrenocortical deficiency states. Their synthetic analogs are primarily used for their potent anti-inflammatory effects in disorders of many organ systems.
Glucocorticoids cause profound and varied metabolic effects. In addition, they modify the body’s immune responses to diverse stimuli.
INDICATIONS AND USAGE
Intra-Articular
Kenalog-10 Injection (Triamcinolone Acetonide Injectable Suspension, USP) is indicated for intra-articular or intrabursal administration, and for injection into tendon sheaths, as adjunctive therapy for short-term administration (to tide the patient over an acute episode or exacerbation) in: synovitis of osteoarthritis, rheumatoid arthritis, acute and subacute bursitis, acute gouty arthritis, epicondylitis, acute nonspecific tenosynovitis, and post-traumatic osteoarthritis.
Intradermal
Intralesional administration of Kenalog-10 Injection is indicated for the treatment of keloids, discoid lupus erythematosus, necrobiosis lipoidica diabeticorum, alopecia areata, and localized hypertrophic, infiltrated, inflammatory lesions of: lichen planus, psoriatic plaques, granuloma annulare, and lichen simplex chronicus (neurodermatitis). Kenalog-10 Injection also may be useful in cystic tumors of an aponeurosis or tendon (ganglia).
CONTRAINDICATIONS
Corticosteroids are contraindicated in patients with systemic fungal infections.
WARNINGS
Because it is a suspension, the preparation should not be administered intravenously. Strict aseptic technique is mandatory.
When patients who are receiving corticosteroid therapy are subjected to unusual stress, increased dosage of rapidly acting corticosteroids is indicated before, during, and after the stressful situation. Kenalog-10 Injection (Triamcinolone Acetonide Injectable Suspension, USP), as a long-acting preparation, is not suitable for use in acute stress situations.
Corticosteroids may mask some signs of infection, and new infections may appear during their use. There may be decreased resistance and inability to localize infection when corticosteroids are used. If an infection occurs during corticosteroid therapy, it should be promptly controlled by suitable antimicrobial therapy (see PRECAUTIONS).
Prolonged use of corticosteroids may produce posterior subcapsular cataracts, glaucoma with possible damage to the optic nerves, and may enhance the establishment of secondary ocular infections due to fungi or viruses.
Average and large doses of hydrocortisone or cortisone can cause elevation of blood pressure, salt and water retention, and increased excretion of potassium. These effects are less likely to occur with the synthetic derivatives except when they are used in large doses; dietary salt restriction and potassium supplementation may be necessary (see PRECAUTIONS). All corticosteroids increase calcium excretion.
Children who are on immunosuppressant drugs are more susceptible to infections than healthy children. Chickenpox and measles, for example, can have a more serious or even fatal course in children on immunosuppressant corticosteroids. In such children, or in adults who have not had these diseases, particular care should be taken to avoid exposure. If exposed, therapy with varicella zoster immune globulin (VZIG) or pooled intravenous immunoglobulin (IVIG), as appropriate, may be indicated. If chickenpox develops, treatment with antiviral agents may be considered.
Patients should not be vaccinated against smallpox while on corticosteroid therapy. Other immunization procedures should not be undertaken in patients who are on corticosteroids, especially on high dose, because of possible hazards of neurological complications and a lack of antibody response.
The use of triamcinolone acetonide in patients with active tuberculosis should be restricted to those cases of fulminating or disseminated tuberculosis in which the corticosteroid is used for the management of the disease in conjunction with an appropriate antituberculous regimen. If corticosteroids are indicated in patients with latent tuberculosis or tuberculin reactivity, close observation is necessary since reactivation of the disease may occur. During prolonged corticosteroid therapy these patients should receive chemoprophylaxis.
Because rare instances of anaphylactoid reactions have occurred in patients receiving parenteral corticosteroid therapy, appropriate precautionary measures should be taken prior to administration, especially when the patient has a history of allergy to any drug.
Safety of use of Kenalog-10 Injection (Triamcinolone Acetonide Injectable Suspension, USP) by intraturbinal, subconjunctival, subtenons, and retrobulbar injection has not been established.
Usage in Pregnancy
Since adequate human reproduction studies have not been done with corticosteroids, the use of these drugs in pregnancy, nursing mothers, or women of child-bearing potential requires that the possible benefits of the drug be weighed against the potential hazards to the mother and the embryo, fetus, or nursing infant. Infants born of mothers who have received substantial doses of corticosteroids during pregnancy should be carefully observed for signs of hypoadrenalism.
PRECAUTIONS
Drug-induced secondary adrenocortical insufficiency may be minimized by a gradual reduction of dosage. This type of relative insufficiency may persist for months after discontinuation of therapy; therefore, in any situation of stress (such as trauma, surgery, or severe illness) occurring during that period, hormone therapy should be reinstituted. Since mineralocorticoid secretion may be impaired, salt and/or a mineralocorticoid should be administered concurrently.
There is an enhanced corticosteroid effect in patients with hypothyroidism and in those with cirrhosis.
Corticosteroids should be used cautiously in patients with ocular herpes simplex because of possible corneal perforation.
The lowest possible dose of corticosteroid should be used to control the condition being treated. A gradual reduction in dosage should be made when possible.
Psychic derangements may appear when corticosteroids are used. These may range from euphoria, insomnia, mood swings, personality changes, and severe depression, to frank psychotic manifestations. Existing emotional instability or psychotic tendencies may also be aggravated by corticosteroids.
Aspirin should be used cautiously in conjunction with corticosteroids in patients with hypoprothrombinemia.
Corticosteroids should be used with caution in patients with nonspecific ulcerative colitis if there is a probability of impending perforation, abscess, or other pyogenic infection. Corticosteroids should also be used cautiously in patients with diverticulitis, fresh intestinal anastomoses, active or latent peptic ulcer, renal insufficiency, hypertension, osteoporosis, and myasthenia gravis.
Patients who are on immunosuppressant doses of corticosteroids should be warned to avoid exposure to chickenpox or measles and, if exposed, to obtain medical advice.
Growth and development of infants and children on prolonged corticosteroid therapy should be carefully observed.
Although therapy with Kenalog-10 Injection (Triamcinolone Acetonide Injectable Suspension, USP) may ameliorate symptoms, it is in no sense a cure and the hormone has no effect on the cause of the inflammation. Therefore, this method of treatment does not obviate the need for the conventional measures usually employed.
Intra-articular injection of a corticosteroid may produce systemic as well as local effects. The inadvertent injection of the suspension into the soft tissues surrounding a joint is not harmful, but is the most common cause of failure to achieve the desired local results.
Following intra-articular steroid therapy, patients should be specifically warned to avoid overuse of joints in which symptomatic benefit has been obtained. Negligence in this matter may permit an increase in joint deterioration that will more than offset the beneficial effects of the steroid. To detect deterioration follow-up x-ray examination is suggested in selected cases.
Overdistention of the joint capsule and deposition of steroid along the needle track should be avoided in intra-articular injection since this may lead to subcutaneous atrophy.
Corticosteroids should not be injected into unstable joints. Repeated intra-articular injection may in some cases result in instability of the joint. In selected cases, particularly when repeated injections are given, x-ray follow-up is suggested.
An increase in joint discomfort has seldom occurred. A marked increase in pain accompanied by local swelling, further restriction of joint motion, fever, and malaise are suggestive of a septic arthritis. If these complications should appear, and the diagnosis of septic arthritis is confirmed, administration of triamcinolone acetonide should be stopped, and antimicrobial therapy should be instituted immediately and continued for 7 to 10 days after all evidence of infection has disappeared. Appropriate examination of any joint fluid present is necessary to exclude a septic process.
Local injection of a steroid into a previously infected joint is to be avoided.
Kenalog-10 Injection should be administered only with full knowledge of characteristic activity of, and varied responses to, adrenocortical hormones. Like other potent corticosteroids, triamcinolone acetonide should be used under close clinical supervision. Triamcinolone acetonide can cause elevation of blood pressure, salt and water retention, and increased potassium and calcium excretion necessitating dietary salt restriction and potassium supplementation. Edema may occur in the presence of renal disease with a fixed or decreased glomerular filtration rate.
During prolonged therapy, a liberal protein intake is essential for counteracting the tendency to gradual weight loss sometimes associated with negative nitrogen balance, wasting and weakness of skeletal muscles.
When local or systemic microbial infections are present, therapy with triamcinolone acetonide is not recommended, but may be employed with caution and only in conjunction with appropriate antibiotic or chemotherapeutic medication. Triamcinolone acetonide may mask signs of infection and enhance dissemination of the infecting organism. Hence, all patients receiving triamcinolone acetonide should be watched for evidence of intercurrent infection. Should infection occur, vigorous, appropriate anti-infective therapy should be initiated. If possible, abrupt cessation of steroids should be avoided because of the danger of superimposing adrenocortical insufficiency on the infectious process.
Menstrual irregularities may occur, and this possibility should be mentioned to female patients past menarche.
In peptic ulcer, recurrence may be asymptomatic until perforation or hemorrhage occurs. X-rays should be taken in peptic ulcer patients complaining of gastric distress, or when therapy is prolonged. Whether or not changes are observed, an ulcer regimen is recommended.
As with other corticosteroids, the possibility of other severe reactions should be considered. If such reactions should occur, appropriate corrective measures should be instituted and use of the drug discontinued.
Continued supervision of the patient after termination of triamcinolone acetonide therapy is essential, since there may be a sudden reappearance of severe manifestations of the disease for which the patient was treated.
ADVERSE REACTIONS
Undesirable reactions following intra-articular administration of the preparation have included postinjection flare, transient pain, occasional local irritation at the injection site, sterile abscesses, hyper- and hypopigmentation, charcot-like arthropathy, and occasional brief increase in joint discomfort; following intradermal administration, rare instances of blindness associated with intralesional therapy around the face and head, transient local discomfort, sterile abscesses, hyper- and hypopigmentation, and subcutaneous and cutaneous atrophy (which usually disappears, unless the basic disease process is itself atrophic) have occurred.
Since systemic absorption may occasionally occur with intra-articular or other local administration, patients should be watched closely for the following adverse reactions which may be associated with any corticosteroid therapy:
Fluid and electrolyte disturbances— sodium retention, fluid retention, congestive heart failure in susceptible patients, potassium loss, cardiac arrhythmias or ECG changes due to potassium deficiency, hypokalemic alkalosis, and hypertension.
Musculoskeletal— muscle weakness, fatigue, steroid myopathy, loss of muscle mass, osteoporosis, vertebral compression fractures, delayed healing of fractures, aseptic necrosis of femoral and humeral heads, pathologic fractures of long bones, and spontaneous fractures.
Gastrointestinal— peptic ulcer with possible subsequent perforation and hemorrhage, pancreatitis, abdominal distention, and ulcerative esophagitis.
Dermatologic— impaired wound healing, thin fragile skin, petechiae and ecchymoses, facial erythema, increased sweating, purpura, striae, hirsutism, acneiform eruptions, lupus erythematosus-like lesions and suppressed reactions to skin tests.
Neurological— convulsions, increased intracranial pressure with papilledema (pseudo-tumor cerebri) usually after treatment, vertigo, headache, neuritis or paresthesias, and aggravation of pre-existing psychiatric conditions.
Endocrine— menstrual irregularities; development of the cushingoid state; suppression of growth in children; secondary adrenocortical and pituitary unresponsiveness, particularly in times of stress (e.g., trauma, surgery, or illness); decreased carbohydrate tolerance; manifestations of latent diabetes mellitus; and increased requirements for insulin or oral hypoglycemic agents in diabetics.
Ophthalmic— posterior subcapsular cataracts, increased intraocular pressure, glaucoma, and exophthalmos.
Metabolic— hyperglycemia, glycosuria, and negative nitrogen balance due to protein catabolism.
Others— necrotizing angiitis, thrombophlebitis, thromboembolism, aggravation or masking of infections, insomnia, syncopal episodes, and anaphylactoid reactions.
DOSAGE AND ADMINISTRATION
Dosage
The initial dose of Kenalog-10 Injection (Triamcinolone Acetonide Injectable Suspension, USP) for intra-articular or intrabursal administration and for injection into tendon sheaths may vary from 2.5 mg to 5 mg for smaller joints and from 5 to 15 mg for larger joints depending on the specific disease entity being treated. Single injections into several joints for multiple locus involvement, up to a total of 20 mg or more, have been given without incident. For intradermal administration, the initial dose of triamcinolone acetonide will vary depending upon the specific disease entity being treated but should be limited to 1.0 mg (0.1 mL) per injection site, since larger volumes are more likely to produce cutaneous atrophy. Multiple sites (separated by one centimeter or more) may be so injected, keeping in mind that the greater the total volume employed the more corticosteroid becomes available for possible systemic absorption and subsequent corticosteroid effects. Such injections may be repeated, if necessary, at weekly or less frequent intervals.
The lower dosages in the initial dosage range of triamcinolone acetonide may produce the desired effect when the corticosteroid is administered to provide a localized concentration. The site of the injection and the volume of the injection should be carefully considered when triamcinolone acetonide is administered for this purpose. The initial dosage should be maintained or adjusted until a satisfactory response is noted. If after a reasonable period of time there is a lack of satisfactory clinical response, Kenalog-10 Injection should be discontinued and the patient transferred to other appropriate therapy. IT SHOULD BE EMPHASIZED THAT DOSAGE REQUIREMENTS ARE VARIABLE AND MUST BE INDIVIDUALIZED ON THE BASIS OF THE DISEASE UNDER TREATMENT AND THE RESPONSE OF THE PATIENT. After a favorable response is noted, the proper maintenance dosage should be determined by decreasing the initial drug dosage in small increments at appropriate time intervals until the lowest dosage which will maintain an adequate clinical response is reached. It should be kept in mind that constant monitoring is needed in regard to drug dosage. Included in the situations which may make dosage adjustments necessary are changes in clinical status secondary to remissions or exacerbations in the disease process, the patient’s individual drug responsiveness, and the effect of patient exposure to stressful situations not directly related to the disease entity under treatment; in this latter situation it may be necessary to increase the dosage of Kenalog-10 Injection (Triamcinolone Acetonide Injectable Suspension, USP) for a period of time consistent with the patient’s condition. If the drug is to be stopped after long-term therapy, it is recommended that it be withdrawn gradually rather than abruptly.
Administration
Shake the vial before use to insure a uniform suspension. Prior to withdrawal, inspect suspension for clumping or granular appearance (agglomeration). An agglomerated product results from exposure to freezing temperatures and should not be used. After withdrawal, inject without delay to prevent settling in the syringe. Careful technique should be employed to avoid the possibility of entering a blood vessel or introducing infection.
Routine laboratory studies, such as urinalysis, two-hour postprandial blood sugar, determination of blood pressure and body weight, and a chest x-ray should be made at regular intervals during prolonged therapy. Upper GI x-rays are desirable in patients with an ulcer history or significant dyspepsia.
For treatment of joints, the usual intra-articular injection technique, as described in standard textbooks, should be followed. If an excessive amount of synovial fluid is present in the joint, some, but not all, should be aspirated to aid in the relief of pain and to prevent undue dilution of the steroid.
With intra-articular or intrabursal administration, and with injection of Kenalog-10 Injection (Triamcinolone Acetonide Injectable Suspension, USP) into tendon sheaths, the use of a local anesthetic may often be desirable. When a local anesthetic is used, its package insert should be read with care and all the precautions connected with its use should be observed. It should be injected into the surrounding soft tissues prior to the injection of the corticosteroid. A small amount of the anesthetic solution may be instilled into the joint.
In treating acute nonspecific tenosynovitis, care should be taken to insure that the injection of Kenalog-10 Injection is made into the tendon sheath rather than the tendon substance. Epicondylitis (tennis elbow) may be treated by infiltrating the preparation into the area of greatest tenderness.
For treatment of dermal lesions, Kenalog-10 Injection (Triamcinolone Acetonide Injectable Suspension, USP) is injected directly into the lesion, i.e., intradermally or sometimes subcutaneously. For accuracy of dosage measurement and ease of administration, it is preferable to employ a tuberculin syringe and a small-bore needle (23 to 25 gauge). Ethyl chloride spray may be used to alleviate the discomfort of the injection.
HOW SUPPLIED
Kenalog-10 Injection (Triamcinolone Acetonide Injectable Suspension, USP) is supplied in 5 mL multiple dose vials (NDC 0003-0494-20) providing 10 mg triamcinolone acetonide per mL.
Storage
Store at room temperature; avoid freezing; protect from light.
Bristol-Myers Squibb Company
Princeton, NJ 08543 USA
Product of Italy
1132761
| KENALOG (triamcinolone acetonide) | |||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
Revised: 03/2006Bristol-Myers Squibb Company