METHIMAZOLE
-
methimazole tablet
Sandoz Inc.
----------
Methimazole Tablets USPDESCRIPTION
Methimazole tablets USP (1-methylimidazole-2-thiol) is a white, crystalline substance that is freely soluble in water. It differs chemically from the drugs of the thiouracil series primarily because it has a 5- instead of a 6-membered ring.
Each tablet contains 5 mg or 10 mg (43.8 μmol or 87.6 μmol) methimazole, an orally administered antithyroid drug.
Each tablet also contains anhydrous lactose, colloidal silicon dioxide, lactose monohydrate, magnesium stearate, pregelatinized starch and talc.
The molecular weight is 114.16, and the molecular formula is C4H6N2S. The structural formula is as follows:
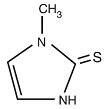
CLINICAL PHARMACOLOGY
Methimazole inhibits the synthesis of thyroid hormones and thus is effective in the treatment of hyperthyroidism. The drug does not inactivate existing thyroxine and triiodothyronine that are stored in the thyroid or circulating in the blood nor does it interfere with the effectiveness of thyroid hormones given by mouth or by injection.
The actions and use of methimazole are similar to those of propylthiouracil. On a weight basis, the drug is at least 10 times as potent as propylthiouracil, but methimazole may be less consistent in action.
Methimazole is readily absorbed from the gastrointestinal tract. It is metabolized rapidly and requires frequent administration. Methimazole is excreted in the urine.
In laboratory animals, various regimens that continuously suppress thyroid function and thereby increase TSH secretion result in thyroid tissue hypertrophy. Under such conditions, the appearance of thyroid and pituitary neoplasms has also been reported. Regimens that have been studied in this regard include antithyroid agents as well as dietary iodine deficiency, subtotal thyroidectomy, implantation of autonomous thyrotropic hormone-secreting pituitary tumors, and administration of chemical goitrogens.
INDICATIONS AND USAGE
Methimazole tablets USP are indicated in the medical treatment of hyperthyroidism. Long-term therapy may lead to remission of the disease. Methimazole tablets USP may be used to ameliorate hyperthyroidism in preparation for subtotal thyroidectomy or methimazole tablets USP radioactive iodine therapy. Methimazole tablets USP are also used when thyroidectomy is contraindicated or not advisable.
CONTRAINDICATIONS
Methimazole is contraindicated in the presence of hypersensitivity to the drug and in nursing mothers because the drug is excreted in milk.
WARNINGS
Agranulocytosis is potentially a serious side effect. Patients should be instructed to report to their physicians any symptoms of agranulocytosis, such as fever or sore throat. Leukopenia, thrombocytopenia, and aplastic anemia (pancytopenia) may also occur. The drug should be discontinued in the presence of agranulocytosis, aplastic anemia (pancytopenia), hepatitis, or exfoliative dermatitis. The patient's bone marrow function should be monitored.
Due to the similar hepatic toxicity profiles of methimazole and propylthiouracil, attention is drawn to the severe hepatic reactions which have occurred with both drugs. There have been rare reports of fulminant hepatitis, hepatic necrosis, encephalopathy, and death. Symptoms suggestive of hepatic dysfunction (anorexia, pruritus, right upper quadrant pain, etc.) should prompt evaluation of liver function. Drug treatment should be discontinued promptly in the event of clinically significant evidence of liver abnormality including hepatic transaminase values exceeding 3 times the upper limit of normal.
Methimazole can cause fetal harm when administered to a pregnant woman. Methimazole readily crosses the placental membranes and can induce goiter and even cretinism in the developing fetus. In addition, rare instances of congenital defects: aplasia cutis, as manifested by scalp defects; esophageal atresia with tracheoesophageal fistula; and choanal atresia with absent/ hypoplastic nipples, have occurred in infants born to mothers who received methimazole during pregnancy. If methimazole is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be warned of the potential hazard to the fetus.
Since the above congenital defects have been reported in offspring of patients treated with methimazole, it may be appropriate to use other agents in pregnant women requiring treatment for hyperthyroidism.
Postpartum patients receiving methimazole should not nurse their babies.
PRECAUTIONS
General
Patients who receive methimazole should be under close surveillance and should be cautioned to report immediately any evidence of illness, particularly sore throat, skin eruptions, fever, headache, or general malaise. In such cases, white-blood-cell and differential counts should be made to determine whether agranulocytosis has developed. Particular care should be exercised with patients who are receiving additional drugs known to cause agranulocytosis.
Laboratory Tests
Because methimazole may cause hypoprothrombinemia and bleeding, prothrombin time should be monitored during therapy with the drug, especially before surgical procedures (see PRECAUTIONS, General).
Periodic monitoring of thyroid function is warranted, and the finding of an elevated TSH warrants a decrease in the dosage of methimazole.
Drug Interactions
Anticoagulants (Oral)
The activity of oral anticoagulants may be potentiated by anti-vitamin-K activity attributed to methimazole.
ß−Adrenergic Blocking Agents
Hyperthyroidism may cause an increased clearance of beta blockers with a high extraction ratio. A dose reduction of beta-adrenergic blockers may be needed when a hyperthyroid patient becomes euthyroid.
Digitalis Glycosides
Serum digitalis levels may be increased when hyperthyroid patients on a stable digitalis glycoside regimen become euthyroid; a reduced dosage of digitalis glycosides may be required.
Theophylline
Theophylline clearance may decrease when hyperthyroid patients on a stable theophylline regimen become euthyroid; a reduced dose of theophylline may be needed.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2 year study, rats were given methimazole at doses of 0.5 mg/kg/day, 3 mg/kg/day and 18 mg/kg/day. These doses were 0.3, 2 and 12 times the 15 mg/day maximum human maintenance dose (when calculated on the basis of surface area). Thyroid hyperplasia, adenoma, and carcinoma developed in rats at the two higher doses. The clinical significance of these findings is unclear.
Pregnancy Category D
Methimazole used judiciously is an effective drug in hyperthyroidism complicated by pregnancy. In many pregnant women, the thyroid dysfunction diminishes as the pregnancy proceeds; consequently, a reduction in dosage may be possible. In some instances, use of methimazole can be discontinued 2 or 3 weeks before delivery (see WARNINGS).
Nursing Mothers
The drug appears in human breast milk and its use is contraindicated in nursing mothers (see WARNINGS).
Pediatric Use
(See DOSAGE AND ADMINISTRATION.)
ADVERSE REACTIONS
Major adverse reactions (which occur with much less frequency than the minor adverse reactions) include inhibition of myelopoiesis (agranulocytosis, granulocytopenia, and thrombocytopenia), aplastic anemia, drug fever, a lupuslike syndrome, insulin autoimmune syndrome (which can result in hypoglycemic coma), hepatitis (jaundice may persist for several weeks after discontinuation of the drug), periarteritis, and hypoprothrombinemia. Nephritis occurs very rarely.
Minor adverse reactions include skin rash, urticaria, nausea, vomiting, epigastric distress, arthralgia, paresthesia, loss of taste, abnormal loss of hair, myalgia, headache, pruritus, drowsiness, neuritis, edema, vertigo, skin pigmentation, jaundice, sialadenopathy, and lymphadenopathy.
It should be noted that about 10% of patients with untreated hyperthyroidism have leukopenia (white-blood-cell count of less than 4,000/mm3), often with relative granulopenia.
OVERDOSAGE
Signs and Symptoms
Symptoms may include nausea, vomiting, epigastric distress, headache, fever, joint pain, pruritus, and edema. Aplastic anemia (pancytopenia) or agranulocytosis may be manifested in hours to days. Less frequent events are hepatitis, nephrotic syndrome, exfoliative dermatitis, neuropathies, and CNS stimulation or depression. Although not well studied, methimazole-induced agranulocytosis is generally associated with doses of 40 mg or more in patients older than 40 years of age.
No information is available on the median lethal dose of the drug or the concentration of methimazole in biologic fluids associated with toxicity and/or death.
Treatment
To obtain up-to-date information about the treatment of overdose, a good resource is your certified Regional Poison Control Center. Telephone numbers of certified poison control centers are listed in the “Physicians’ Desk Reference (PDR)”. In managing overdosage, consider the possibility of multiple drug overdoses, interaction among drugs, and unusual drug kinetics in your patient.
Protect the patient's airway and support ventilation and perfusion. Meticulously monitor and maintain, within acceptable limits, the patient's vital signs, blood gases, serum electrolytes, etc. The patient's bone marrow function should be monitored. Absorption of drugs from the gastrointestinal tract may be decreased by giving activated charcoal, which, in many cases, is more effective than emesis or lavage; consider charcoal instead of or in addition to gastric emptying. Repeated doses of charcoal over time may hasten elimination of some drugs that have been absorbed. Safeguard the patient's airway when employing gastric emptying or charcoal.
Forced diuresis, peritoneal dialysis, hemodialysis, or charcoal hemoperfusion have not been established as beneficial for an overdose of methimazole.
DOSAGE AND ADMINISTRATION
Methimazole tablets USP are administered orally. Tablets are usually given in 3 equal doses at approximately 8-hour intervals.
Adults
The initial daily dosage is 15 mg for mild hyperthyroidism, 30 mg to 40 mg for moderately severe hyperthyroidism and 60 mg for severe hyperthyroidism, divided into 3 doses at 8-hour intervals. The maintenance dosage is 5 mg to 15 mg daily.
Pediatric
Initially, the daily dosage is 0.4 mg/kg of body weight divided into 3 doses and given at 8-hour intervals. The maintenance dosage is approximately 1/2 of the initial dose.
HOW SUPPLIED
Methimazole Tablets USP for oral administration are available as:
5 mg: White, round, biconvex, beveled tablets, scored on one side and debossed “E” over “205” on the other side and supplied as:
NDC 0185-0205-01 bottles of 100
NDC 0185-0205-10 bottles of 1000
10 mg: White, round, biconvex, beveled tablets, scored on one side and debossed “E” over “210” on the other side and supplied as:
NDC 0185-0210-01 bottles of 100
NDC 0185-0210-10 bottles of 1000
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Dispense contents in a tight, light-resistant container as defined in the USP with a child-resistant closure, as required.
KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN.
To report SUSPECTED ADVERSE REACTIONS, contact Sandoz Inc. at 1-800-525-8747 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Sandoz Inc.
Princeton, NJ 08540
OS7631
Rev. 03/10
MF0205REV03/10
MG #16035
Methimazole Tablets USP, 5 mg x 100 Tablets - Label
NDC 0185-0205-01
Methimazole Tablets USP
5 mg
Rx only
100 Tablets
Sandoz

Methimazole Tablets USP, 10 mg x 100 Tablets - Label
NDC 0185-0210-01
Methimazole Tablets USP
10 mg
Rx only
100 Tablets
Sandoz

| METHIMAZOLE
methimazole tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA040411 | 03/27/2001 | |
| METHIMAZOLE
methimazole tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA040411 | 03/27/2001 | |
| Labeler - Sandoz Inc. (614842560) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Sandoz Inc | 614842560 | RELABEL, REPACK, MANUFACTURE, ANALYSIS | |