KIT FOR THE PREPARATION OF TECHNETIUM TC 99M SULFUR COLLOID INJECTION
-
sulfur colloid
Pharmalucence, Inc.
----------
Kit for the Preparation of Technetium Tc 99m Sulfur Colloid Injection Diagnostic for Intravenous and Oral UseDESCRIPTION
Kit for the Preparation of Technetium Tc 99m Sulfur Colloid Injection is a multidose reaction vial with a Solution A vial and a Solution B vial which contain the sterile non-pyrogenic, non-radioactive ingredients necessary to produce Technetium Tc 99m Sulfur Colloid Injection for diagnostic use by intravenous injection or oral administration.
Each 10 mL multidose reaction vial contains, in lyophilized form 2.0 mg sodium thiosulfate anhydrous, 2.3 mg edetate disodium and 18.1 mg gelatin; a Solution A vial with 1.8 mL of 0.148 N hydrochloric acid solution and a Solution B vial with 1.8 mL aqueous solution of 24.6 mg/mL sodium biphosphate anhydrous and 7.9 mg/mL sodium hydroxide.
When a solution of sterile and non-pyrogenic Sodium Pertechnetate Tc 99m Injection in isotonic saline is mixed with these components, following the instructions provided with the kit, Technetium Tc 99m Sulfur Colloid Injection is formed. The product so derived is intended for intravenous injection or oral administration. The precise structure of Technetium Tc 99m Sulfur Colloid Injection is not known at this time.
Technetium Tc 99m decays by isomeric transition with a physical half-life of 6.02 hours.1 The principal photon that is useful for detection and imaging studies is listed in Table 1.
| Radiation | Mean Percent Per Disintegration | Mean Energy (keV) |
| Gamma-2 | 89.07 | 140.5 |
1Kocher DC: Radioactive decay data tables. DOE/TIC-11026: 108, 1981
External Radiation
The specific gamma ray constant for Tc 99m is 0.78 R/millicurie-hr at 1cm. The first half-value layer is 0.017 cm of lead (Pb). A range of values for the relative attenuation of the radiation emitted by this radionuclide that results from interposition of various thicknesses of Pb is shown in Table 2. For example, the use of a 0.25 cm thickness of Pb will attenuate the radiation emitted by a factor of about 1,000.
| Shield Thickness (Pb) cm | Coefficient of Attenuation |
| 0.017 | 0.5 |
| 0.08 | 10-1 |
| 0.16 | 10-2 |
| 0.25 | 10-3 |
| 0.33 | 10-4 |
To correct for physical decay of this radionuclide, the fractions that remain at selected intervals after the time of calibration are shown in Table 3.
| Hours | Fraction Remaining | Hours | Fraction Remaining |
| 0* 1 2 3 4 5 | 1.000 0.891 0.794 0.708 0.631 0.562 | 6 7 8 9 10 11 12 | 0.501 0.447 0.398 0.355 0.316 0.282 0.251 |
*Calibration time
CLINICAL PHARMACOLOGY
Following intravenous administration, Technetium Tc 99m Sulfur Colloid Injection is rapidly cleared by the reticuloendothelial system from the blood with a nominal clearance half-life of approximately 2 1/2 minutes. Uptake of the radioactive colloid by organs of the reticuloendothelial system is dependent upon both their relative blood flow rates and the functional capacity of the phagocytic cells. In the average patient 80 to 90% of the injected collodial particles are phagocytized by the Kupffer cells of the liver, 5 to 10% by the spleen and the balance by the bone marrow.
Following administration of Technetium Tc 99m Sulfur Colloid Injection by intraperitoneal injection, the radiopharmaceutical mixes with the peritoneal fluid. Clearance from the peritoneal cavity varies from insignificant, which may occur with complete shunt blockage, to very rapid clearance with subsequent transfer into the systemic circulation when the shunt is patent.
Serial images should be obtained of both the shunt and liver (target organ). However, an adequate evaluation of the difference between total blockage of the shunt and partial blockage may not be feasible in all cases.
INDICATIONS AND USAGE
Technetium Tc 99m Sulfur Colloid Injection is used in adults and children as an agent for imaging areas of functioning retriculoendothelial cells in the liver, spleen and bone marrow.
It is used orally in adults and children for esophageal transit studies, gastroesophageal reflux scintigraphy, and for the detection of pulmonary aspiration of gastric contents.
Technetium Tc 99m Sulfur Colloid may be used in adults as an imaging agent to aid in the evaluation of peritoneo-venous (LeVeen) shunt patency.
GERIATRIC USE
Clinical studies of Kit for the Preparation of Technetium Tc 99m Sulfur Colloid Injection did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and may be useful to monitor renal function.
CONTRAINDICATIONS
None known.
WARNINGS
Although rare, deaths have occurred following intravenously administered gelatin stabilized Technetium Tc 99m Sulfur Colloid Injection, advanced cardiopulmonary life support systems should be readily available where and when the drug is administered.
PRECAUTIONS
General
The contents of the two Solution vials, the Solution A vial containing the appropriate acidic solution and the Solution B vial containing the appropriate buffer solution, are intended only for use in the preparation of the Technetium Tc 99m Sulfur Colloid Injection and are NOT to be directly administered to the patient.
Sodium Pertechnetate Tc 99m Injection containing oxidants should not be used to reconstitute this kit.
The contents of the kit are not radioactive. However, after the Sodium Pertechnetate Tc 99m Injection is added, adequate shielding of the final preparation must be maintained.
The contents of the kit are sterile and non-pyrogenic. It is essential to follow the directions carefully and to adhere strictly to aseptic procedures during preparation.
This preparation contains no bacteriostatic preservative.
The stability of the colloidal preparation may be decreased in the presence of polyvalent cations, thus resulting in the agglomeration of the individual colloidal particles. These larger particles are likely to be trapped by the pulmonary capillary bed following intravenous injection.
Sodium Pertechnetate Tc 99m Injection containing more than 10 micrograms per milliliter of aluminum ion should not be used to formulate the Technetium Tc 99m Sulfur Colloid Injection.
Technetium Tc 99m Sulfur Colloid Injection is physically unstable, and the particles will settle with time. Failure to agitate the vial adequately before use may result in non-uniform distribution of radioactivity. Because of the increasing probability of agglomeration with aging, it is recommended that a vial of the prepared finished drug should not be used more than six hours from the time of formulation.
The components of the Kit for the Preparation of Technetium Tc 99m Sulfur Colloid Injection are supplied sterile and non-pyrogenic. Aseptic procedures normally employed in making additions and withdrawals for sterile, non-pyrogenic containers should be used during addition of the pertechnetate solution and the withdrawal of doses for patient administration.
No special handling is required for the non-radioactive drug product.
Radiopharmaceuticals should be used only by physicians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
As in the use of any other radioactive material, care should be taken to minimize radiation exposure to patient and clinical personnel, consistent with proper patient management.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate carcinogenic potential or whether Technetium Tc 99m Sulfur Colloid Injection affects fertility in males or females. Mutagenesis studies have not been conducted.
Pregnancy
Animal reproduction and teratogenicity studies have not been conducted with Technetium Tc 99m Sulfur Colloid Injection. It is also not known whether Technetium Tc 99m Sulfur Colloid Injection can cause fetal harm when administered to a pregnant woman, or can affect reproductive capacity. There have been no studies in pregnant women. Technetium Tc 99m Sulfur Colloid Injection should be given to a pregnant woman only if clearly needed.
Ideally, examinations using radiopharmaceuticals, especially those elective in nature, of a woman of childbearing capability, should be performed during the first few (approximately 10) days following the onset of menses.
Nursing Mothers
Technetium Tc 99m is excreted in human milk during lactation. Therefore, formula feeding should be substituted for breast feeding
ADVERSE REACTIONS
The following adverse reactions have been reported associated with the use of Technetium Tc 99m Sulfur Colloid Injection: cardiopulmonary arrest, seizures, anaphylactic shock, hypotension, dyspnea, abdominal pain, fever, chills, bronchospasm, nausea, vomiting, perspiration, redness, urticaria, numbness, dizziness and burning at the injection site.
Several deaths and cases of lung and soft tissue uptake other than RES have been reported in association with the use of Technetium Tc 99m Sulfur Colloid Injection
(see WARNINGS).
The size and physical-chemical properties of the sulfur colloid particles formed from the components of the kit may determine the biodistribution of the colloid and its uptake by the RE system. Diseases affecting the RE system may also alter the expected uptake pattern.
DOSAGE AND ADMINISTRATION
Shielding should be utilized when preparing Technetium Tc 99m Sulfur Colloid Injection.
Liver-Spleen Scanning and Bone Marrow Imaging:
In average ADULT (70 kg) patients:
The suggested dose range used for liver/spleen images is 37 to 296 megabecquerels
(1 to 8 millicuries) and for bone marrow images is 111 to 444 megabecquerels (3 to 12 millicuries) of Technetium Tc 99m Sulfur Colloid Injection.
In PEDIATRIC patients:
The suggested intravenous doses employed for various diagnostic indications are as follows:
Liver/spleen imaging: The dose range is 0.56 to 2.78 megabecquerels (15 to 75 microcuries) per kilogram of body weight with a usual dose of 1.85 megabecquerels (50 microcuries) per kg body weight, except in newborns in whom the administered dose should be 7.4 to 18.5 megabecquerels (200 to 500 microcuries). A minimum dose of 7.4 megabecquerels (200 microcuries) should be employed for this procedure.
Bone marrow imaging: The dose range is 1.11 to 5.55 megabecquerels (30 to 150 microcuries) per kilogram of body weight. A minimum dose of 22.2 megabecquerels (600 microcuries) is suggested for this procedure.
(See Table 5)
Gastroesophageal and Pulmonary Aspiration:
In the average ADULT (70kg) patients:
The suggested oral dose range is 5.55-11.1 megabecquerels (150-300 microcuries) for gastroesophageal studies. The suggested oral dose range for pulmonary aspiration studies is
11.1-18.5 megabecquerels (300-500 microcuries).
In PEDIATRIC patients:
The suggested oral dose range in infants and children is 3.7-11.1 megabecquerels (100-300 microcuries) for gastroesophageal and pulmonary aspiration studies.
The drug should be incorporated into a milk feeding when administered orally. Equally good results may be obtained by intubating the stomach and directly instilling the material into the stomach, followed by a dextrose or milk meal. This latter method avoids the introduction of radiation into the esophagus; thus, any tracer appearing there must be due to reflux.
The patient dose should be measured by a suitable radioactivity calibration system immediately prior to administration.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
Peritoneo-venous (LeVeen) Shunt Patency Evaluation:
The suggested intraperitoneal dosage range used in the average patient (70 kg) for peritoneo-venous (LeVeen) shunt patency evaluation is 37 to 111 megabecquerels (1 to 3 millicuries). Adequate measures should be taken to assure uniform mixing with peritoneal fluid. Serial images of both the shunt and target organ should be obtained and correlated with other clinical findings. Alternatively, the drug may be administered by percutaneous transtubal injection. The suggested percutaneous transtubal (efferent limb) dosage range for the average patient (70 kg) is 12 to 37 megabecquerels (0.3 to 1.0 millicurie) in a volume not to exceed 0.5 mL.
Transperitoneal absorption of Sulfur Colloid may occur, but it occurs slowly. Therefore, the most definitive scintigraphic evaluation of shunt patency will be obtained if there is visualization of both the shunt itself and the liver and/or spleen within the first three hours post intraperitoneal injection.
Radiation Dosimetry
The estimated absorbed radiation doses2 to an average ADULT patient (70 kg) from an intravenous injection of a maximum dose of 296 megabecquerels (8 millicuries) of Technetium Tc 99m Sulfur Colloid Injection are shown in Table 4.
| Technetium Tc 99m, Sulfur Colloid Injection, mGy/296 MBq (rads/8mCl) | |||
| Diffuse Parenchymal Disease | |||
| Target Organ | Normal Liver | Early Intermediate | Intermediate Advanced |
| Liver | 27 (2.7) | 17 (1.7) | 13 (1.3) |
| Spleen | 17 (1.7) | 22 (2.2) | 34 (3.4) |
| Bone Marrow | 2.2 (0.22) | 3.6 (0.36) | 6.3 (0.63) |
| Testes | 0.088 (0.0088) | 0.17 (0.017) | 0.26 (0.026) |
| Ovaries | 0.45 (0.045) | 0.65 (0.065) | 0.96 (0.096) |
| Total Body | 1.5 (0.15) | 1.5 (0.15) | 1.4 (0.14) |
2Modified from Summary of Current Radiation Dose Estimates to Humans with Various Liver Conditions from 99m Tc-Sulfur Colloid, MIRD Dose Estimate Report No 3, J Nucl Med 16: 108A - 108B, 1975
PEDIATRIC RADIATION DOSES
In PEDIATRIC patients, the radiation absorbed doses, using the maximum recommended doses for liver/spleen imaging [2.78 megabecquerels (75 microcuries) per kilogram of body weight] and for bone marrow imaging [5.55 megabecquerels (150 microcuries) per kilogram of body weight], except in the newborn where the doses used for calculating the radiation absorbed dose estimates are 18.5 megabecquerels (500 microcuries) for liver/spleen imaging and 22.2 megabecquerels (600 microcuries) for bone marrow imaging, are shown in Table 5.
| Age | Newborn | 1 year | 5 years | 10 years | 15 years | |||||
| Weight (kg) | 3.5 | 12.1 | 20.3 | 33.5 | 55.0 | |||||
| Maximum | ||||||||||
| Recommended Dose | ||||||||||
| megabecquerels | 18.5a | 22.2b | 33.3a | 67.3b | 55.5a | 114.7b | 92.5a | 186.1b | 151.7a | 307.1b |
| (millicuries) | (0.5)a | (0.6)b | (0.9)a | (1.82)b | (1.5)a | (3.1)b | (2.5)a | (5.03)b | (4.1)a | (8.3)b |
| Absorbed Dose | ||||||||||
| mGy/max dose | ||||||||||
| (rads/max dose) | ||||||||||
| Total body | 0.6 (0.06)a | 0.71 (0.071) | 0.86 (0.086) | 1.73 (0.173) | 0.99 (0.099) | 2.09 (0.209) | 1.07 (0.107) | 2.16 (0.216) | 0.9 (0.09) | 1.83 (0.183) |
| Liver | 16 (1.6) | 19 (1.9) | 12.6 (1.26) | 25.5 (2.55) | 12.3 (1.23) | 25.4 (2.54) | 16.7 (1.67) | 33.2 (3.32) | 20.1 (2.01) | 40.7 (4.07) |
| Spleen | 14 (1.4) | 17 (1.7) | 10.8 (1.08) | 21.8 (2.18) | 9.75 (0.975) | 20.2 (2.02) | 12.2 (1.22) | 24.7 (2.47) |
13.5 (1.35) | 27.4 (2.74) |
| Red Marrow | 2.9 (0.29) | 3.5 (0.35) | 1.62 (0.162) | 32.8 (3.28) | 1.65 (0.165) | 34.1 (3.41) | 2.03 (0.203) | 4.07 (0.407) | 1.48 (0.148) | 2.99 (0.299) |
| Ovaries | 0.7 (0.07) | 0.81 (0.081) | 0.58 (0.058) | 1.17 (0.117) | 0.57 (0.057) | 1.08 (0.108) | 0.4 (0.04) | 0.81 (0.081) | 0.34 (0.034) | 0.69 (0.069) |
| Testes | 0.2 (0.02) | 0.71 (0.071) | 0.19 (0.019) | 0.38 (0.038) | 0.2 (0.02) | 0.4 (0.04) | 0.35 (0.035) | 0.70 (0.070) | 0.09 (0.009) | 0.18 (0.018) |
Assumptions:
1. Used the biologic data of MIRD Dose Estimate Report No 3 J Nucl Med 16: 108A - 108B, 1975
2. Used the Age-dependent “S” values of Henrichs et al, Berlin 1980, except for the 1-year old. The 1-year old “S” values were taken from preliminary phantom work of the Metabolism and Dosimetry Group at ORNL.
a Liver/Spleen Imaging Dose (see DOSAGE AND ADMINISTRATION).
b Bone Marrow Imaging Dose (see DOSAGE AND ADMINISTRATION).
| Target Organ | Assumed Residence Time (hr.) | (mGy/18.5 MBq) | Absorbed Dose (mrad/500 uci) |
| Total Body Stomach Wall Small Intestine Upper Large Intestine Wall Lower Large Intestine Wall Ovaries Testes | - 1.5 4 13 24 - - | 0.09 0.07 1.30 2.40 1.65 0.48 0.025 | 9 70 130 240 165 48 2.5 |
RADIATION DOSES TO HOSPITAL PERSONNEL
| Technician | Preparation of Drug* | Administered Drug |
| Target | µSv/7.4 GBq (millirem/400 mCi) | µSv/148 MBq (millirem/8 mCi) |
| Extremity Dose | 230 (23) | 20 (2) |
| Whole Body Dose | 10 (1) | 1 (0.1) |
*Using shielded vial and syringe.
| Target Organ | Shunt Patency (Open) | Shunt Patency (Closed) |
||
| mGy | rads | mGy | rads | |
| Liver Ovaries & Testes Organs in the Peritoneal Cavity Total Body | 10.2 0.036 to 0.18 - 0.54 | 1.02 0.0036 to 0.018 - 0.054 | 1.68 1.68 1.68 0.57 | 0.168 0.168 0.168 0.057 |
Assumptions:
Calculations for the absorbed radiation dose are based upon an effective half-time of 3 hours for the open shunt and 6.02 hours for the closed shunt and an even distribution of the radiopharmaceutical in the peritoneal cavity with no biological clearance.
HOW SUPPLIED
Kit for the Preparation of Technetium Tc 99m Sulfur Colloid Injection is supplied as a 5-kit package. Five complete kits are included in each package. All components are sterile and non-pyrogenic. Each 10mL multidose reaction vial contains, in lyophilized form, 2.0 mg sodium thiosulfate anhydrous, 2.3 mg edetate disodium and 18.1 mg gelatin; each Solution A vial contains 1.8 mL 0.148 N hydrochloric acid solution and each Solution B vial contains 1.8 mL aqueous solution of 24.6 mg/mL sodium biphosphate anhydrous and 7.9 mg/mL sodium hydroxide. Included in each 5-kit package are one package insert and 10 radiation labels. Store the kit as packaged at 15-30°C.
Directions for Use
Technetium Tc 99m Sulfur Colloid Injection is prepared by the following aseptic procedure:
1. Waterproof gloves should be worn during the preparation procedure. Remove the dark brown plastic cap from the Sulfur Colloid reaction vial and swab the top of the vial closure with alcohol to sterilize the surface.
2. Complete the radiation label and affix to the vial. Place the vial in an appropriate lead-capped radiation shield labeled and identified.
With a sterile shielded syringe, aseptically obtain 1-3mL of a suitable, oxidant-free sterile and non-pyrogenic Sodium Pertechnetate Tc 99m Injection, each milliliter containing a maximum activity of 18.5 gigabecquerels (500 millicuries). Do not use Sodium Pertechnetate Tc 99m Injection if it contains foreign matter or more than 10 micrograms/mL of aluminum (Sodium Pertechnetate Tc 99m Injection containing more than a total of 10 micrograms/mL of aluminum may produce a flocculent precipitate and since such a precipitate may localize in the lung, preparations containing precipitates should not be used).
3. Aseptically add the Sodium Pertechnetate Tc 99m Injection to the vial.
4. Place a lead cover on the vial shield and dissolve the reagent by gentle swirling.
5. Just prior to use, remove the red cap from the Solution A vial and swab the top of the vial closure with alcohol to sterilize the surface. Using a sterile needle and syringe, aseptically withdraw 1.5 mL Solution A from the vial. Aseptically Inject 1.5mL Solution A into the reaction vial and swirl again.
6. Transfer the reaction vial from vial shield and place in a vigorously boiling water bath (water bath should be shielded with 1/8” to 1/4” lead) deep enough to cover the entire liquid contents of the vial. Keep the vial in the water bath for five minutes.
7. Remove the reaction vial from the water bath and place in the lead shield and allow to cool for three minutes. Swab the vial closure again with an antiseptic.
8. Just prior to use, remove the blue cap from the Solution B vial and swab the top of the vial closure with alcohol to sterilize the surface. Using a sterile needle and syringe, aseptically withdraw 1.5 mL Solution B from the vial. Aseptically Inject 1.5 mL Solution B into the reaction vial and swirl again.
9. Record time and date of preparation.
10. Allow the preparation to cool to body temperature before use. Maintain adequate shielding of the radioactive colloid preparation at all times.
11. Where appropriate, dilute the preparation with sterile Sodium Chloride Injection
to bring the dosage to within the recommended range.
12. The radiochemical purity of the prepared radiopharmaceutical should be checked prior to patient administration.
13. Mix the reaction vial and aseptically withdraw material with a sterile shielded syringe for use within six (6) hours of preparation. For optimum results this time should be minimized. The vial contains no bacteriostatic preservative. Store the reconstituted vial at 15-30°C. Discard vial six (6) hours after reconstitution.
14. The patient dose should be measured by a suitable radioactivity calibration system immediately prior to administration.
NDC # 45567-0030-1
This reagent kit for preparation of a radiopharmaceutical is approved for use by persons licensed pursuant to Section 120.547, Code of Massachusetts Regulation 105, or under equivalent license of the U.S. Nuclear Regulatory Commission or an Agreement State.
Manufactured by
Pharmalucence, Inc.
10 DeAngelo Drive
Bedford, MA 01730
RM 2A-067
03/08
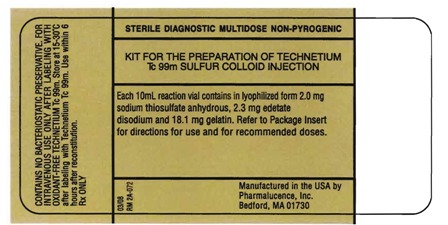
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - VIAL CONTAINER (PART 1 - 10mL Reaction Vial)
NDC 045567-0030-1
Sterile Diagnostic Multidose Non-Pyrogenic
Kit for the Preparation of Technetium Tc 99m Sulfur Colloid Injection
Each 10mL reaction vial contains in lyophilized form 2.0 mg sodium thiosulfate anhydrous, 2.3 mg edetate disodium and 18.1 mg gelatin. Refer to Package Insert for directions for use and for recommended doses.
Manufactured in the USA by
Pharmalucence, Inc.
Bedford, MA 01730
CONTAINS NO BACTERIOSTATIC PRESERVATIVE . FOR INTRAVENOUS USE ONLY AFTER LABELING WITH OXIDANT-FREE TECHNETIUM Tc 99m. Store at 15-30°C after labeling with Technetium Tc 99m. Use within 6 hours after reconstitution.
PACKAGE/LABEL - PRINCIPAL DISPLAY PANEL - V1AL CONTAINER (PART 2 - 3mL Solution A Vial)
NDC 045567-0030-1
A
Solution A vial contains 1.8mL sterile, non-pyrogenic 0.148N hydrochloric acid solution. To be used only with the Sulfur Colloid Reaction Vial.
NOT FOR DIRECT INTRAVENOUS INJECTION.
RX ONLY. STORE AT 15-30°C
Manufactured By: Pharmalucence, Inc. Bedford, MA 01730 U.S.A.

PACKAGE/LABEL - PRINCIPAL DISPALY PANEL - VIAL CONTAINER (PART 3 - 3mL Solution B Vial)
NDC 045567-0030-1
B
Solution B vial contains 1.8mL sterile, non-pyrogenic aqueous solution of 24.6 mg/ml sodium biphosphate anhydrous and 7.9 mg/ml sodium hydroxide. To be used only with the Sulfur Colloid Reaction Vial.
NOT FOR DIRECT INTRAVENOUS INJECTION.
RX ONLY. STORE AT 15-30°C
Manufactured By: Pharmalucence, Inc. Bedford, MA 01730 U.S.A.

PACKAGE/LABEL - PRINCIPAL DISPLAY PANEL - 5 VIAL BOX
NDC 045567-0030-1
Kit for the Preparation of Technetium Tc99m Sulfur Colloid Injection
CAUTION: Federal (U.S.A.) law prohibits dispensing without prescription
Rx only.
Manufactured By:
Pharmalucence, Inc.
10 DeAngelo Drive, Bedford, MA 01730
For Customer Service call: 1-800-221-7554
Sterile Diagnostic Multidose Non-Pyrogenic
CONTENTS: 1 package insert, 10 radiation labels, 5 reaction vials, 5 solution A vials and 5 solution B vials. Each 10 mL reaction vial contains 2.0 mg sodium thiosulfate anhydrous, 2.3 mg edetate disodium and 18.1 mg gelatin. Each Solution A vial contains 1.8 mL 0.148 N hydrochloric acid. Each solution B vial contains 1.8 mL aqueous solution of 24.6 mg/mL sodium biphosphate anhydrous and 7.9 mg/mL sodium hydroxide. Contains no bacteriostatic preservative. For intravenous use only after labeling with oxidant-free Technetium Tc 99m.
STORE KIT AS PACKAGED AT 15-30°C
Store reconstituted vials at 15-30°C. Use within 6 hours after labeling with Technetium
Tc 99m.
Refer to Package Insert for recommended adult doses.
IMPORTANT: Read enclosed Package Insert for full information on preparation, use and indications.

| KIT FOR THE PREPARATION OF TECHNETIUM TC 99M SULFUR COLLOID INJECTION
sulfur colloid kit |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA017858 | 04/19/1978 | |
| Labeler - Pharmalucence, Inc. (139261648) |
| Registrant - Pharmalucence, Inc. (139261648) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Pharmalucence, Inc. | 139261648 | MANUFACTURE, ANALYSIS | |