ISOSORBIDE DINITRATE
-
isosorbide dinitrate tablet, extended release
Amedra Pharmaceutical
----------
IsoDitrateTM Extended-Release Tablets, USP(isosorbide dinitrate)
40 mg
Rx only
DESCRIPTION
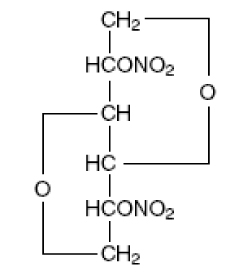
Isosorbide Dinitrate an organic nitrate, is a vasodilator with effects on both arteries and veins. IsoDitrate (brand name for isosorbide dinitrate) is available as 40 mg Extended-Release Tablets. The chemical name for Isosorbide Dinitrate 1,4: 3,6- dianhydro- D- glucitol 2, 5- dinitrate, an organic nitrate whose structural formula is and whose molecular weight is 236.14. The organic nitrates are vasodilators, active on both arteries and veins.
Isosorbide Dinitrate is a white, crystalline, odorless compound which is stable in air and in solution, has a melting point of 70°C and has an optical rotation of +134° (c=1.0, alcohol, 20°C). Isosorbide Dinitrate is freely soluble in organic solvents such as acetone, alcohol, and ether, but is only sparingly soluble in water.
Each IsoDitrate Extended-Release Tablet, (brand of isosorbide dinitrate) for oral administration, contains 40 mg of Isosorbide Dinitrate, in a matrix that causes the active drug to be released over a sustained period. In addition, each tablet also contains the following inactive ingredients: colloidal silicon dioxide, hypromellose, lactose monohydrate and magnesium stearate.
Conformance of Isosorbide Dinitrate Extended-release Tablets with USP release test is pending.
CLINICAL PHARMACOLOGY
The principal pharmacological action of isosorbide dinitrate is relaxation of vascular smooth muscle and consequent dilatation of peripheral arteries and veins, especially the latter. Dilatation of the veins promotes peripheral pooling of blood and decreases venous return to the heart, thereby reducing left ventricular end-diastolic pressure and pulmonary capillary wedge pressure (preload). Arteriolar relaxation reduces systemic vascular resistance, systolic arterial pressure, and mean arterial pressure (afterload). Dilatation of the coronary arteries also occurs. The relative importance of preload reduction, afterload reduction, and coronary dilatation remains undefined.
Dosing regimens for most chronically used drugs are designed to provide plasma concentrations that are continuously greater than a minimally effective concentration. This strategy is inappropriate for organic nitrates. Several well-controlled clinical trials have used exercise testing to assess the anti-anginal efficacy of continuously-delivered nitrates. In the large majority of these trials, active agents were no more effective than placebo after 24 hours (or less) of continuous therapy. Attempts to overcome nitrate tolerance by dose escalation, even to doses far in excess of those used acutely, have consistently failed. Only after nitrates have been absent from the body for several hours has their anti-anginal efficacy been restored.
Pharmacokinetics
The kinetics of absorption of isosorbide dinitrate has not been well studied. Studies of immediate-release formulations of isosorbide dinitrate have found highly variable bioavailability (10% to 90%) with extensive first-pass metabolism in the liver. Most such studies have observed progressive increases in bioavailability during chronic therapy: it is not known whether similar increases in bioavailability appear during the course of chronic therapy with Isosorbide Dinitrate Extended-Release Tablets.
Once absorbed, the volume of distribution of isosorbide dinitrate is 2 to 4 L/kg, and this volume is cleared at the rate of 2 to 4 L/min, so Isosorbide Dinitrate's half-life in serum is about an hour. Since the clearance exceeds hepatic blood flow, considerable extrahepatic metabolism must also occur. Clearance is effected primarily by denitration to the 2-mononitrate (15 to 25%) and the 5-mononitrate (75 to 85%).
Both metabolites have biological activity, especially the 5-mononitrate. With an overall half-life of about 5 hours, the 5-mononitrate is cleared from the serum by denitration to isosorbide, glucuronidation to the 5-mononitrate glucuronide, and denitration/hydration to sorbitol. The 2-mononitrate has been less well studied, but it appears to participate in the same metabolic pathways, with a half-life of about 2 hours.
The daily dose-free interval sufficient to avoid tolerance to Iisosorbide dinitrate has not been well defined. Studies of nitroglycerin (an organic nitrate with a very short half-life) have shown that daily dose-free intervals of 10 to 12 hours are usually sufficient to minimize tolerance. Daily dose-free intervals that have succeeded in avoiding tolerance during trials of moderate doses (e.g., 30 mg) of immediate-release isosorbide dinitrate have generally been somewhat longer (at least 14 hours), but this is consistent with the longer half-lives of isosorbide dinitrate and its active metabolites. A dose-free interval sufficient to avoid tolerance with Isosorbide Dinitrate Extended-Release Tablets has not been demonstrated. Clinical trials using Isosorbide Dinitrate Extended-Release Tablets in a regimen designed to avoid tolerance have not been conducted, but in a multiple-dose study of another controlled-release Isosorbide Dinitrate product, 40 mg capsules were administered at 0800 and 1400 hours. After two weeks of this regimen, the controlled-release Isosorbide Dinitrate product was statistically indistinguishable from placebo. For the formulation of controlled-release Isosorbide Dinitrate that was tested, the necessary dose-free interval must therefore be greater than 18 hours; the necessary interval for Isosorbide Dinitrate Extended-Release Tablets remains unknown.
Few well-controlled clinical trials of organic nitrates have been designed to detect rebound or withdrawal effects. In one such trial, however, subjects receiving nitroglycerin had less exercise tolerance at the end of the daily dose-free interval than the parallel group receiving placebo. The incidence, magnitude, and clinical significance of similar phenomena in patients receiving isosorbide dinitrate have not been studied.
Clinical trials
In clinical trials, immediate-release oral isosorbide dinitrate has been administered in a variety of regimens, with total daily doses ranging from 30 mg to 480 mg. Controlled trials of single oral doses of controlled-release isosorbide dinitrate have demonstrated effective reductions in exercise-related angina for up to 8 hours. Anti-anginal activity is present about 1 hour after dosing.
Adequate multiple-dose trials of Isosorbide Dinitrate Extended-Release Tablets have not been reported.
Most controlled trials of multiple-dose immediate-release oral isosorbide dinitrate taken every 12 hours (or more frequently) for several weeks have shown statistically significant anti-anginal efficacy for only 2 hours after dosing. Once-daily regimens, and regimens with one daily dose-free interval of at least 14 hours (e.g., a regimen providing doses at 0800, 1400 and 1800 hours), have shown efficacy after the first dose of each day that was similar to that shown in the single-dose studies cited above. The efficacy of subsequent doses has not been demonstrated.
From large, well-controlled studies of other nitrates, it is reasonable to believe that the maximal achievable daily duration of anti-anginal effect from Isosorbide ditrate is about 12 hours. No dosing regimen for Isosorbide Dinitrate Extended-Release Tablets has, however, ever actually been shown to achieve this duration of effect.
INDICATIONS AND USAGE
Isosorbide Dinitrate Extended-Release Tablets are indicated for the prevention of angina pectoris due to coronary artery disease. The onset of action of controlled-release oral isosobide dinitrate is not sufficiently rapid for this product to be useful in aborting an acute anginal episode.
CONTRAINDICATIONS
Allergic reactions to organic nitrates are extremely rare, but they do occur. Isosorbide Dinitrate Extended-Release Tablets are contraindicated in patients who are allergic to isosorbide dinitrate or to any of its other ingredients.
WARNINGS
Amplification of the vasodilatory effects of IsoDitrate (isosorbide dinitrate) by sildenafil can result in severe hypotension. The time course and dose dependence of this interaction have not been studied. Appropriate supportive care has not been studied, but it seems reasonable to treat this as a nitrate overdose, with elevation of the extremities and with central volume expansion.
The benefits of controlled-release oral isosorbide dinitrate in patients with acute myocardial infarction or congestive heart failure have not been established. If one elects to use isosorbide dinitrate in these conditions, careful clinical or hemodynamic monitoring must be used to avoid the hazards of hypotension and tachycardia. Because the effects of controlled-release oral isosorbide dinitrate are so difficult to terminate rapidly, this formulation is not recommended in these settings.
PRECAUTIONS
General
Severe hypotension, particularly with upright posture, may occur with even small doses of isosorbide dinitrate. This drug should therefore be used with caution in patients who may be volume depleted or who, for whatever reason, are already hypotensive. Hypotension induced by isosorbide dinitrate may be accompanied by paradoxical bradycardia and increased angina pectoris.
Nitrate therapy may aggravate the angina caused by hypertrophic cardiomyopathy.
As tolerance to isosorbide dinitrate develops, the effect of sublingual nitroglycerin on exercise tolerance, although still observable, is somewhat blunted.
Some clinical trials in angina patients have provided nitroglycerin for about 12 continuous hours of every 24-hour day. During the daily dose-free interval in some of these trials, anginal attacks have been more easily provoked than before treatment, and patients have demonstrated hemodynamic rebound and decreased exercise tolerance. The importance of these observations to the routine, clinical use of controlled-release oral isosorbide dinitrate is not known.
In industrial workers who have had long-term exposure to unknown (presumably high) doses of organic nitrates, tolerance clearly occurs. Chest pain, acute myocardial infarction, and even sudden death have occurred during temporary withdrawal of nitrates from these workers, demonstrating the existence of true physical dependence.
Information for Patients
Patients should be told that the anti-anginal efficacy of IsoDitrate (isosorbide dinitrate) is strongly related to its dosing regimen, so the prescribed schedule of dosing should be followed carefully. In particular, daily headaches sometimes accompany treatment with IsoDitrate (isosorbide dinitrate). In patients who get these headaches, the headaches are a marker of the activity of the drug. Patients should resist the temptation to avoid headaches by altering the schedule of their treatment with IsoDitrate (isosorbide dinitrate), since loss of headache may be associated with simultaneous loss of anti-anginal efficacy. Aspirin and/or acetaminophen, on the other hand, often successfully relieve isosorbide dinitrate-induced headaches with no deleterious effect an IsoDitrate's (isosorbide dinitrate) anti-anginal efficacy.
Treatment with IsoDitrate (isosorbide dinitrate) may be associated with lightheadedness on standing, especially just after rising from a recumbent or seated position. This effect may be more frequent in patients who have also consumed alcohol.
Drug Interactions
The vasodilating effects of IsoDitrate (isosorbide dinitrate) may be additive with those of other vasodilators. Alcohol, in particular, has been found to exhibit additive effects of this variety.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies in animals have been performed to evaluate the carcinogenic potential of IsoDitrate (isisorbide dinitrate). In a modified two-liter reproduction study, there was no remarkable gross pathology and no altered fertility or gestation among rats fed IsoDitrate (isosorbide dinitrate) at 25 or 100 mg/kg/day.
Pregnancy Category C
At oral doses 35 and 150 times the maximum recommended human daily dose, IsoDitrate (isosorbide dinitrate) has been shown to cause a dose-related increase in embryotoxicity (increase in mummified pups) in rabbits. There are no adequate, well-controlled studies in pregnant women. IsoDitrate (isosorbide dinitrate) should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether IsoDitrate (isosorbide dinitrate) is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when IsoDitrate (Isosorbide dinitrate) is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
Clinical studies of IsoDitrate (isosorbide dinitrate) did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
Adverse reactions to IsoDitrate (isosorbide dinitrate) are generally dose-related, and almost all of these reactions are the result of IsoDitrate's (isosorbide dinitrate) activity as a vasodilator. Headache, which may be severe, is the most commonly reported side effect. Headache may be recurrent with each daily dose, especially at higher doses. Transient episodes of lightheadedness, occasionally related to blood pressure changes, may also occur. Hypotension occurs infrequently, but in some patients it may be severe enough to warrant discontinuation of therapy. Syncope, crescendo angina, and rebound hypertension have been reported but are uncommon.
Extremely rarely, ordinary doses of organic nitrates have caused methemoglobinemia in normal-seeming patients. Methemoglobinemia is so infrequent at these doses that further discussion of its diagnosis and treatment is deferred (see “OVERDOSAGE”).
Data are not available to allow estimation of the frequency of adverse reactions during treatment with IsoDitrate (isosorbide dinitrate) Extended-Release Tablets.
OVERDOSAGE
Hemodynamic Effects
The ill effects of IsoDitrate (isosorbide ditrate) overdose are generally the results of IsoDitrate's (isosorbide dinitrate) capacity to induce vasodilatation, venous pooling, reduced cardiac output, and hypotension. These hemodynamic changes may have protean manifestations, including increased intracranial pressure, with any or all of persistent throbbing headache, confusion, and moderate fever; vertigo; palpitations; visual disturbances; nausea and vomiting (possibly with colic and even bloody diarrhea); syncope (especially in the upright posture); air hunger and dyspnea, later followed by reduced ventilatory effort; diaphoresis, with the skin either flushed or cold and clammy; heart block and bradycardia, paralysis; coma; seizures; and death.
Laboratory determinations of serum levels of IsoDitrate (isosorbide dinitrate) and its metabolites are not widely available, and such determinations have, in any event, no established role in the management of IsoDitrate (isosorbide dinitate) overdose.
There are no data suggesting what dose of IsoDitrate (isosorbide dinitrate) is likely to be life-threatening in humans. In rats, the median acute lethal dose (LD50) was found to be 1100 mg/kg.
No data are available to suggest physiological maneuvers (e.g., maneuvers to change the pH of the urine) that might accelerate elimination of IsoDitrate (isosorbide dinitrate) and its active metabolites. Similarly, it is not known which, if any, of these substances can usefully be removed from body by hemodialysis.
No specific antagonist to the vasodilator effect of IsoDitrate (isosorbide dinitrate) is known, and no intervention has been subject to controlled study as a therapy for Isosorbide Dinitrate overdose. Because the hypotension associated with IsoDitrate (isosorbide dinitrate) overdose is the result of venodilatation and arterial hypovolemia, prudent therapy in this situation should be directed toward increase in central fluid volume. Passive elevation of the patient’s legs may be sufficient, but intravenous infusion of normal saline or similar fluid may also be necessary.
The use of epinephrine or other arterial vasoconstrictors in this setting is likely to do more harm than good.
In patients with renal disease or congestive heart failure, therapy resulting in central volume expansion is not without hazard. Treatment of IsoDitrate (isosorbide dinitrate) overdosage in these patients may be subtle and difficult, and invasive monitoring may be required.
Methemoglobinemia
Nitrate ions liberated during metabolism of IsoDitrate (isosorbide dinitrate) can oxidize hemoglobin into methemoglobin. Even in patients totally without cytochrome b5 reductase activity, however, and even assuming that the nitrate moieties of IsoDitrate (isosorbide dinitrate) are quantitatively applied to oxidation of hemoglobin, about 1 mg/kg of IsoDitrate (isosorbide dinitrate) should be required before any of these patients manifests clinically significant (≤ 10%) methemoglobinemia. In patients with normal reductase function, significant production of methemoglobin should require even larger doses of IsoDitrate (isosorbide dinitrate). In one study in which 36 patients received 2 to 4 weeks of continuous nitroglycerin therapy at 3.1 to 4.4 mg/ hr (equivalent, in total administered dose of nitrate ions, to 4.8 to 6.9 mg of bioavailable IsoDitrate (isosorbide dinitrate) per hour), the average methemoglobin level measured was 0.2%; this was comparable to that observed in parallel patients who received placebo.
Notwithstanding these observations, there are case reports of significant methemoglobinemia in association with moderate overdoses of organic nitrates. None of the patients had been thought to be unusually susceptible.
Methemoglobin levels are available from most clinical laboratories. The diagnosis should be suspected in patients who exhibit signs of impaired oxygen delivery despite adequate cardiac output and adequate arterial pO2. Classically, methemoglobinemic blood is described as chocolate brown, without color change on exposure to air.
When methemoglobinemia is diagnosed, the treatment of choice is methylene blue, 1 to 2 mg/kg intravenously.
DOSAGE AND ADMINISTRATION
As noted under “CLINICAL PHARMACOLOGY”, multiple-dose studies with isosorbide dinitrate and other nitrates have shown that maintenance of continuous 24-hour plasma levels results in refractory tolerance. Every dosing regimen for IsoDitrate (isosorbide dinitrate) Extended-Release Tablets must provide a daily dose-free interval to minimize the development of this tolerance. With immediate-release Iisosorbide dinitrate, it appears that one daily dose-free interval must be at least 14 hours long. The necessary dose-free interval for IsoDitrate (isosorbide dinitrate) Extended-Release Tablets has not been clearly identified, but is presumably somewhat longer.
As also noted under “CLINICAL PHARMACOLOGY”, only one trial has ever studied the use of controlled-release isosorbide dinitrate for more than one dose. In that trial, 40 mg of a different formulation of controlled-release Iisosorbide dinitrate was administered twice daily in doses given 6 hours apart. After 4 weeks, active treatment could not be distinguished from placebo.
Large controlled studies with other nitrates suggest that no dosing regimen with IsoDitrate (isosorbide dinitrate) Extended-Release Tablets should be expected to provide more than about 12 hours of continuous anti-anginal efficacy per day.
In clinical trials, immediate-release oral isosorbide dinitrate has been administered in a variety of regimens, with total daily doses ranging from 30 mg to 480 mg.
HOW SUPPLIED
Isosorbide Dinitrate Extended-Release Tablets, USP 40 mg are available as white, round, scored compressed tablets, debossed cor above the bisect and 183 below the bisect and the other side is plain. They are supplied as follows:
Bottles of 100 (NDC 52054-183-10)
STORAGE STATEMENT
Store at 20o to 25oC (68o to 77o). [See USP Controlled Room Temperature].
Dispense in a tight light container as defined in the USP.
Keep this and all drugs out of the reach of children.
Manufactured by:
Corepharma, LLC
Middlesex, NJ 08846
Distributed by:
Amedra Pharmaceuticals, LLC
Middlesex, NJ 08846
MF#781 Rev. April, 2010
PRODUCT LABEL
Product Label
NDC 52054-183-10
IsoDitrate ER Tablets
(Isosorbide Dinitrate, USP)
40 mg
100 Extended Release Tablets
Rx Only amedra pharmaceuticals

| ISOSORBIDE DINITRATE
isosorbide dinitrate tablet, extended release |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA040723 | 04/02/2008 | |
| Labeler - Amedra Pharmaceutical (961668824) |