FLUTICASONE PROPIONATE
-
fluticasone propionate ointment
Physicians Total Care, Inc.
----------
Rx only
For Dermatologic Use Only—Not for Ophthalmic Use.
DESCRIPTION
Fluticasone propionate ointment, 0.005% contains fluticasone propionate [(6α,11β,16α,17α)-6,9,-difluoro-11-hydroxy-16-methyl-3-oxo-17-(1-oxopropoxy)androsta-1,4-diene-17-carbothioic acid, S-fluoromethyl ester], a synthetic fluorinated corticosteroid, for topical dermatologic use. The topical corticosteroids constitute a class of primarily synthetic steroids used as anti-inflammatory and antipruritic agents.
Chemically, fluticasone propionate is C25H31F3O5S. It has the following structural formula:
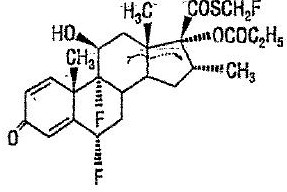
Fluticasone propionate has a molecular weight of 500.6. It is a white to off-white powder and is insoluble in water.
Each gram of fluticasone propionate ointment, 0.005% contains fluticasone propionate 0.05 mg in a base of liquid paraffin, microcrystalline wax, propylene glycol, and sorbitan sesquioleate.
CLINICAL PHARMACOLOGY
Like other topical corticosteroids, fluticasone propionate has anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory activity of the topical steroids, in general, is unclear. However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor, arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2.
Fluticasone propionate is lipophilic and has a strong affinity for the glucocorticoid receptor. It has weak affinity for the progesterone receptor, and virtually no affinity for the mineralocorticoid, estrogen, or androgen receptors. The therapeutic potency of glucocorticoids is related to the half-life of the glucocorticoid-receptor complex. The half-life of the fluticasone propionate-glucocorticoid receptor complex is approximately 10 hours.
Studies performed with fluticasone propionate ointment, 0.005% indicate that it is in the medium range of potency as compared with other topical corticosteroids.
PharmacokineticsAbsorptionThe activity of fluticasone propionate ointment, 0.005% is due to the parent drug, fluticasone propionate. The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle and the integrity of the epidermal barrier. Occlusive dressing enhances penetration. Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin increase percutaneous absorption.
In a study of 6 healthy volunteers applying 25 g of fluticasone propionate ointment 0.005% twice daily to the trunk and legs for up to 5 days under occlusion, plasma levels of fluticasone ranged from 0.08 to 0.22 ng/mL.-
In an animal study using radiolabeled 0.05% fluticasone propionate cream and ointment preparations, rats received a topical dose of 1 g/kg for a 24-hour period. Total recovery of radioactivity was approximately 80% at the end of 7 days. The majority of the dose (73%) was recovered from the surface of the application site. Less than 1% of the dose was recovered in the skin at the application site. Approximately 5% of the dose was absorbed systemically through the skin. Absorption from the skin continued for the duration of the study (7 days), indicating a long retention time at the application site.
DistributionFollowing intravenous administration of 1 mg of fluticasone propionate in healthy volunteers, the initial disposition phase for fluticasone propionate was rapid and consistent with its high lipid solubility and tissue binding. The apparent volume of distribution averaged 4.2 L/kg (range, 2.3-16.7 L/kg). The percentage of fluticasone propionate bound to human plasma proteins averaged 91%. Fluticasone propionate is weakly and reversibly bound to erythrocytes. Fluticasone propionate is not significantly bound to human transcortin.
MetabolismNo metabolites of fluticasone propionate were detected in an in vitro study of radiolabeled fluticasone propionate incubated in a human skin homogenate. The total blood clearance of systemically absorbed fluticasone propionate averages 1093 mL/min (range, 618-1702 mL/min) after a 1-mg intravenous dose, with renal clearance accounting for less than 0.02% of the total. Fluticasone propionate is metabolized in the liver by cytochrome P450 3A4-mediated hydrolysis of the 5-fluoromethyl carbothioate grouping. This transformation occurs in 1 metabolic step to produce the inactive 17-β-carboxylic acid metabolite, the only known metabolite detected in man. This metabolite has approximately 2000 times less affinity than the parent drug for the glucocorticoid receptor of human lung cytosol in vitro and negligible pharmacological activity in animal studies. Other metabolites detected in vitro using cultured human hepatoma cells have not been detected in man.
ExcretionFollowing an intravenous dose of 1 mg in healthy volunteers, fluticasone propionate showed polyexponential kinetics and had an average terminal half-life of 7.2 hours (range, 3.2-11.2 hours).
INDICATIONS AND USAGE
Fluticasone propionate ointment, 0.005% is a medium potency corticosteroid indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses in adult patients.
CONTRAINDICATIONS
Fluticasone propionate ointment, 0.005% is contraindicated in those patients with a history of hypersensitivity to any of the components in the preparation.
PRECAUTIONS
Enter section text here
ADVERSE REACTIONS
In controlled clinical trials, the total incidence of adverse reactions associated with the use of fluticasone propionate ointment, 0.005% was approximately 4%. These adverse reactions were usually mild, self-limiting, and consisted primarily of pruritus, burning, hypertrichosis, increased erythema, hives, irritation, and lightheadedness. Each of these events occurred individually in less than 1% of patients. In a study of 35 pediatric patients treated with fluticasone propionate ointment 0.005% for atopic dermatitis over at least 35% of body surface area, subnormal adrenal function was observed with cosyntropin stimulation testing at the end of 3 to 4 weeks of treatment in 4 patients who had normal testing prior to treatment. It is not known if these patients had recovery of adrenal function because follow-up testing was not performed (see PRECAUTIONS:Pediatric Use, and ADVERSE REACTIONS). Telangiectasia on the face was noted in one patient on the eighth day of a four week treatment period. Facial use was discontinued and the telangiectasia resolved.
The following additional local adverse reactions have been reported infrequently with topical corticosteroids, including fluticasone propionate, and they may occur more frequently with the use of occlusive dressings and higher potency corticosteroids. These reactions are listed in an approximately decreasing order of occurrence: dryness, folliculitis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, skin atrophy, striae, and miliaria. Also, there are discontinuation of potent topical corticosteroid products.
OVERDOSAGE
Topically applied fluticasone propionate ointment, 0.005% can be absorbed in sufficient amounts to produce systemic effects (see PRECAUTIONS).
DOSAGE AND ADMINISTRATION
Apply a thin film of fluticasone propionate ointment, 0.005% to the affected skin areas twice daily. Rub in gently.
Fluticasone propionate ointment, 0.005% should not be used with occlusive dressings.
Geriatric UseIn studies where geriatric patients (65 years of age or older, see PRECAUTIONS) have been treated with fluticasone propionate ointment, 0.005%, safety did not differ from that in younger patients; therefore, no dosage adjustment is recommended.
HOW SUPPLIED
fluticasone propionate ointment, 0.005% is supplied in:
60-g tubes - NDC 54868-5458-0
Store between 2° and 30°C (35° and 86°F).
Mfg. By: CLAY-PARK LABS, INC. Bronx, NY 10457
I221
January 2003
Relabeling of "Additional Barcode" by:
Physicians Total Care, Inc.
Tulsa, OK 74146
PRINCIPAL DISPLAY PANEL
Fluticasone Propionate Ointment, 0.005%

| FLUTICASONE PROPIONATE
fluticasone propionate ointment |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA076668 | 10/20/2005 | |
| Labeler - Physicians Total Care, Inc. (194123980) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Physicians Total Care, Inc. | 194123980 | relabel | |