YF-VAX
-
yellow fever virus live antigen, a
Sanofi Pasteur Inc.
----------
YELLOW FEVER VACCINEYF-VAX®
AHFS Category 80:12
Rx only
DESCRIPTION
YF-VAX®, Yellow Fever Vaccine, for subcutaneous use, is prepared by culturing the 17D-204 strain of yellow fever virus in living avian leukosis virus-free (ALV-free) chicken embryos. The vaccine contains sorbitol and gelatin as a stabilizer, is lyophilized, and is hermetically sealed under nitrogen. No preservative is added. The vaccine must be reconstituted immediately before use with the sterile diluent provided (Sodium Chloride Injection USP – contains no preservative). YF-VAX vaccine is formulated to contain not less than 4.74 log10 plaque forming units (PFU) per 0.5 mL dose throughout the life of the product. YF-VAX vaccine is a slight pink-brown suspension after reconstitution.
CLINICAL PHARMACOLOGY
Yellow fever is an acute viral illness caused by a mosquito-borne flavivirus. The clinical spectrum of yellow fever is highly variable, from subclinical infection to overwhelming pansystemic disease. Yellow fever has an abrupt onset after an incubation period of 3 to 6 days, and usually includes fever, prostration, headache, photophobia, lumbosacral pain, extremity pain (especially the knee joints), epigastric pain, anorexia, and vomiting. The illness may progress to liver and renal failure, and hemorrhagic symptoms and signs caused by thrombocytopenia and abnormal clotting and coagulation may occur. The case-fatality rate of yellow fever varies widely in different studies and may be different for Africa compared to South America, but is typically 20% or higher. Jaundice or other gross evidence of severe liver disease is associated with higher mortality rates. (1)
Two live, attenuated yellow fever vaccines, strains 17D-204 and 17DD, were derived in parallel in the 1930s. Historical data suggest that these "17D vaccines" have identical safety and immunogenicity profiles. Despite a marked reduction in the world-wide incidence of yellow fever in the last five decades due to the extensive use of 17D vaccines and mosquito eradication programs, at least seven tropical South American countries (Bolivia, Brazil, Colombia, Ecuador, French Guiana, Peru, and Venezuela) and much of sub-Saharan Africa (2) currently experience yellow fever epidemics. However, the actual areas of yellow fever virus activity far exceed the infected zones officially reported for epidemics. Approximately 200,000 yellow fever cases have been reported to occur world-wide each year. Six fatalities from yellow fever were reported between 1996 and July 2002, among unimmunized American and European travelers who visited rural areas within the yellow fever endemic zone. (3) (4) (5) (6) (7) (8)
Vaccination with 17D strain viruses is predicted to elicit an immune response identical in quality to that induced by wild-type infection. This response is presumed to result from initial infection of cells in the dermis or other subcutaneous tissues near the injection site, with subsequent replication and limited spread of virus leading to the processing and presentation of viral antigens to the immune system, as would occur during infection with wild-type yellow fever virus. The humoral immune response to the viral structural proteins, as opposed to a cell-mediated response, is most important in the protective effect induced by 17D vaccines. Yellow fever antibodies with specificities that prevent or abort infection of cells are detected as neutralizing antibodies in assays that measure the ability of serum to reduce plaque formation in tissue culture cells. The titer of virus neutralizing antibodies in sera of vaccinees is a surrogate for efficacy. A log10 neutralization index (LNI, measured by a plaque reduction assay) of 0.7 or greater was shown to protect 90% of monkeys from lethal intracerebral challenge. (9) This is the definition of seroconversion adopted for clinical trials of yellow fever vaccine. The standard has also been adopted by the World Health Organization (WHO) for efficacy of yellow fever vaccines in humans. (10)
The neutralizing antibody response to 17D vaccines has been evaluated in several uncontrolled studies since the late 1930s. In 24 studies conducted world-wide between 1962 and 1997 using 17D vaccines involving a total of 2,529 adults and 991 infants and children, the seroconversion rate was greater than 91% in all but two studies and never lower than 81%. There were no significant age-related differences in immunogenicity. (1)
Five of these 24 studies were conducted in the US between 1962 and 1993 and included 208 adults who received YF-VAX vaccine. The seroconversion rate was 81% in one study involving 32 subjects and 97% to 100% in the other four studies. (11) (12) (13) (14) (15)
In 2001, YF-VAX vaccine was used as a control in a double-blind, randomized comparison trial with another 17D-204 vaccine, conducted at nine centers in the US. YF-VAX vaccine was administered to 725 adults ≥18 years old with a mean age of 38 years. Three hundred twelve of these subjects who received YF-VAX vaccine were evaluated serologically, and 99.3% of them seroconverted with a mean LNI of 2.21. The LNI was slightly higher among males compared to females and slightly lower among Hispanic and African-American subjects compared to others, but these differences were not significant with respect to the protective effect of the vaccine. There was no difference in mean LNI for subjects <40 years old compared to subjects ≥40 years old. Due to the small number of subjects (1.7%) with prior flavivirus immunity, it was not possible to draw conclusions about the role of this factor in the immune response. (16)
Results of one clinical trial involving 33 HIV-positive adults residing in the US indicate that the seroconversion rate to 17D-204 vaccine may be reduced in these patients. (17)
In pregnancy or in immunosuppressed individuals the seroconversion rate after administration of yellow fever vaccine may be significantly reduced. (18)
Existing data suggest that the small percentage of immunologically normal subjects who fail to develop an immune response to an initial vaccination may do so upon re-vaccination. (19)
In two separate clinical trials of 17D-204 vaccines, 90% of subjects seroconverted within 10 days after vaccination, (20) and 100% of subjects seroconverted within 14 days. (11) Thus, International Health regulations stipulate that the vaccination certificate for yellow fever is valid 10 days after administration of YF-VAX vaccine. (21)
INDICATIONS AND USAGE
YF-VAX vaccine is recommended for active immunization of persons 9 months of age and older in the following categories:
Persons Living in or Traveling to Endemic Areas
While the actual risk for contracting yellow fever during travel is probably low, variability of itineraries and behaviors and the seasonal incidence of disease make it difficult to predict the actual risk for a given individual traveling to a known endemic or epidemic area. Persons greater than or equal to 9 months of age traveling to or living in areas of South America and Africa where yellow fever infection is officially reported at the time of travel should be vaccinated. Vaccination is also recommended for travel outside the urban areas of countries that do not officially report the disease but that lie in a yellow fever endemic zone.
International Travel
Yellow fever vaccination may be required for international travel. Some countries in Africa require evidence of vaccination from all entering travelers and some countries may waive the requirements for travelers staying less than 2 weeks that are coming from areas where there is no current evidence of significant risk for contracting yellow fever. Some countries require an individual, even if only in transit, to have a valid International Certificate of Vaccination if the individual has been in countries either known or thought to harbor yellow fever virus. The certificate becomes valid 10 days after vaccination with YF-VAX vaccine. (2) (21)
In no instance should infants less than 9 months of age receive yellow fever vaccine, because of the risk of encephalitis (see CONTRAINDICATIONS and ADVERSE REACTIONS sections).
Laboratory Personnel
Those laboratory personnel who might be exposed to virulent yellow fever virus or to concentrated preparations of the yellow fever vaccine strain by direct or indirect contact or by aerosols should be vaccinated. (2)
As with any vaccine, vaccination with YF-VAX vaccine may not protect 100% of individuals (see CLINICAL PHARMACOLOGY section).
For concomitant administration with other vaccines see PRECAUTIONS section, Drug Interactions subsection.
CONTRAINDICATIONS
Hypersensitivity
YF-VAX vaccine is contraindicated in anyone with a history of acute hypersensitivity reaction to any components (including gelatin). (22) Because the yellow fever virus used in the production of this vaccine is propagated in chicken embryos, YF-VAX vaccine should not be administered to anyone with a history of acute hypersensitivity to eggs or egg products; anaphylaxis may occur. Less severe or localized manifestations of allergy to eggs or to feathers are not contraindications to vaccine administration and do not usually warrant vaccine skin testing (see PRECAUTIONS section, Hypersensitivity Reactions subsection). Generally, persons who are able to eat eggs or egg products may receive the vaccine.(2) (23)
Acute or Febrile Disease
Vaccination should be postponed in case of an acute or febrile disease; a disease with low-grade fever is usually not a reason to postpone vaccination.
Infants
Vaccination of infants less than 9 months of age IS CONTRAINDICATED because of the risk of encephalitis, and travel of such persons to rural areas in yellow fever endemic zones or to countries experiencing an epidemic should be postponed or avoided, whenever possible.
Immunosuppressed Patients
Exposure to yellow fever vaccine, which is a live virus vaccine, poses a risk of encephalitis or other serious adverse events to patients with illnesses that commonly result in immunosuppression (eg, acquired immunodeficiency syndrome or other manifestations of human immunodeficiency virus (HIV) infection, leukemia, lymphoma, thymic disease, generalized malignancy), or patients whose immunologic responses are suppressed by drug therapy (eg, corticosteroids, alkylating drugs, or antimetabolites) or radiation. There is evidence suggesting that thymic dysfunction is an independent risk factor for the development of yellow fever vaccine-associated viscerotropic disease, and health care providers should be careful to ask about a history of thymus disorder, including myasthenia gravis, thymoma or prior thymectomy. (24)
Immunosuppressed subjects should not be immunized, and travel to yellow fever endemic areas should be postponed or avoided. If travel to a yellow fever-infected zone is unavoidable, immunosuppressed patients should be advised of the risk, instructed in methods for avoiding vector mosquitoes, and supplied with vaccination waiver letters by their physicians (see ADVERSE REACTIONS section). Family members of immunosuppressed persons, who themselves have no contraindications, may receive yellow fever vaccine. (2) (25)
Lactation
Lactation: (See PRECAUTIONS section, Nursing Mothers subsection.)
WARNINGS
The stopper of the vial contains dry natural latex rubber that may cause allergic reactions.
Anaphylaxis may occur following the use of YF-VAX vaccine, even in individuals with no prior history of hypersensitivity to the vaccine components.
EPINEPHRINE INJECTION (1:1000) SHOULD ALWAYS BE IMMEDIATELY AVAILABLE IN CASE OF AN UNEXPECTED ANAPHYLACTIC OR OTHER SERIOUS ALLERGIC REACTION.
Yellow fever vaccines must be considered as a possible, but rare, cause of vaccine-associated viscerotropic disease (2) (previously described as multiple organ system failure), (2) (26) that is similar to fulminant yellow fever caused by wild-type yellow fever virus. Available evidence suggests that the occurrence of this syndrome may depend upon the presence of undefined host factors, rather than intrinsic virulence of the yellow fever strain 17D vaccine viruses isolated from subjects with vaccine-associated viscerotropic disease. (26) (27) (28) (29) (See ADVERSE REACTIONS section.)
Vaccine-associated neurotropic disease (2), previously described as post-vaccinal encephalitis (1), is a known rare adverse event associated with yellow fever vaccination. Age less than 9 months and immunosuppression are known risk factors for this adverse event. (See CONTRAINDICATIONS and ADVERSE REACTIONS sections.)
PRECAUTIONS
General
Prior to an injection of any vaccine, all known precautions should be taken to prevent adverse events. The patient's previous immunization history, current health status, and medical history should be reviewed for previous hypersensitivity reactions and other adverse events related to this vaccine or similar vaccines and for possible sensitivity to dry natural latex rubber. The stopper of the vial contains dry natural latex rubber that may cause allergic reactions. In some instances where symptoms appear soon after a vaccine is administered, differentiation between allergic reaction to the vaccine and reaction to an environmental allergen may not be possible. (23)
EPINEPHRINE INJECTION (1:1000) SHOULD ALWAYS BE IMMEDIATELY AVAILABLE IN CASE OF AN UNEXPECTED ANAPHYLACTIC OR OTHER SERIOUS ALLERGIC REACTION.
A separate, sterile syringe and needle or a sterile disposable unit should be used for each patient to prevent transmission of blood borne infectious agents. Needles should not be recapped and should be disposed of according to biohazard waste guidelines.
Hypersensitivity Reactions
YF-VAX vaccine should not be administered to an individual with a history of hypersensitivity to egg or chicken protein (see CONTRAINDICATIONS section). However, if a subject is suspect as being an egg-sensitive individual, the following test can be performed before the vaccine is administered: (23)
- 1.
- Scratch, prick, or puncture test: Place a drop of a 1:10 dilution of the vaccine in physiologic saline on a superficial scratch, prick, or puncture on the volar surface of the forearm. Positive (histamine) and negative (physiologic saline) controls should also be used. The test is read after 15 to 20 minutes. A positive test is a wheal 3 mm larger than that of the saline control, usually with surrounding erythema. The histamine control must be positive for valid interpretation. If the result of this test is negative, an intradermal (ID) test should be performed.
- 2.
- Intradermal test: Inject a dose of 0.02 mL of a 1:100 dilution of the vaccine in physiologic saline. Positive and negative control skin tests should be performed concurrently. A wheal 5 mm or larger than the negative control with surrounding erythema is considered a positive reaction.
If vaccination is considered essential, despite a positive skin test, then desensitization can be considered (see DOSAGE AND ADMINISTRATION section, Desensitization subsection).
Information for Patients
Prior to administration of YF-VAX vaccine, potential vaccinees or their parents or guardians should be asked about their recent health status. All potential vaccinees or their parents or guardians should be fully informed of the benefits and risks of immunization and potential for adverse events that have been temporally associated with YF-VAX vaccine administration. Vaccinees or their parents or guardians should be instructed to report all serious adverse events that occur up to 30 days post-vaccination to their health-care provider.
All travelers should seek information regarding vaccination requirements by consulting local health departments, the Centers for Disease Control and Prevention (CDC), and WHO. Travel agencies, international airlines, and/or shipping lines may also have up-to-date information. Such requirements may be strictly enforced, particularly for persons traveling from Africa or South America to Asia. Travelers should consult the latest published version of Health Information for International Travel to determine requirements and regulations for vaccination. (25)
An International Certificate of Vaccination should be completed, signed, and validated with the center's stamp where the vaccine is administered and provided to all vaccinees. The immunization record should contain the date, lot number and manufacturer of the vaccine administered. (30) (31) (32) Subjects should be told that US vaccination certificates are valid for a period of 10 years commencing 10 days after initial vaccination or revaccination.
Drug Interactions
Data are limited in regard to the interaction of YF-VAX vaccine with other vaccines.
- Measles (Schwartz strain) vaccine, diphtheria and tetanus toxoids and pertussis vaccine adsorbed (DTP), (33) Hepatitis A and Hepatitis B vaccines, (2) (12) (34) (35) meningococcal vaccine, Menomune® – A/C/Y/W-135, and typhoid vaccine, Typhim Vi®, (2) (12) (34) have been administered with yellow fever vaccine at separate injection sites.
- No data exist on possible interference between yellow fever and rabies or Japanese encephalitis vaccines. (2)
- In a prospective study, persons given 5 cc of commercially available immune globulin did not experience alterations in immunologic responses to the yellow fever vaccine. (2) (36)
- The anti-malarial drug chloroquine has been administered with yellow fever vaccine. (2) (37)
Patients on Corticosteroid Therapy
Oral Prednisone or other systemic corticosteroid therapy may have an immunosuppressive effect on recipients of yellow fever vaccine that potentially decreases immunogenicity and increases the risk of adverse events (see CONTRAINDICATIONS section). Intra-articular, bursal, or tendon injections with Prednisone or other corticosteroids should not constitute an increased hazard to recipients of yellow fever vaccine.
Patients with Asymptomatic HIV Infection
Subjects with asymptomatic HIV infection who have had recent laboratory verification of adequate immune system function and who cannot avoid potential exposure to yellow fever virus should be offered the choice of vaccination. Vaccinees should be monitored for possible adverse effects. The seroconversion rate to 17D vaccines is likely to be reduced in these patients. (17) Therefore, documentation of a protective antibody response is recommended before travel. (See CLINICAL PHARMACOLOGY section.) For discussion of this subject and for documentation of the immune response to vaccine where it is deemed essential, the CDC may be contacted 1-970-221-6400.
Carcinogenesis, Mutagenesis, Impairment of Fertility
YF-VAX vaccine has not been evaluated for its carcinogenic or mutagenic potential or its effect on fertility.
Pregnancy Category C
Animal reproduction studies have not been conducted with YF-VAX vaccine. It is also not known whether YF-VAX vaccine can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. YF-VAX vaccine should be given to a pregnant woman only if clearly needed. The seroconversion rate to 17D vaccines is markedly reduced in pregnant women. (See CLINICAL PHARMACOLOGY section.) (18) For discussion of this subject and for documentation of a protective immune response to vaccine where it is deemed essential, the CDC may be contacted at 1-970-221-6400.
Nursing Mothers
As there is a theoretical risk of transmission of vaccine components to the infants from breast-feeding mothers, lactation constitutes a contraindication, particularly when infants are below 9 months of age because of the risk of encephalitis. (See CONTRAINDICATIONS section.) The risks and benefit should therefore be assessed before making the decision as to whether to immunize a nursing woman. (2)
Pediatric Use
Vaccination of infants less than 9 months of age IS CONTRAINDICATED because of the risk of encephalitis. (See CONTRAINDICATIONS and ADVERSE REACTIONS sections.)
Geriatric Use
Vaccination of subjects greater than 65 years of age should be limited to individuals who are traveling to or reside in known yellow fever endemic or epidemic areas, because of the increased risk for systemic adverse events in this age group. When vaccination is deemed necessary, the health status of such individuals should be evaluated prior to vaccination. Additionally, if vaccinated, elderly subjects should be carefully monitored for adverse events for 10 days post-vaccination (see ADVERSE REACTIONS section). (24) (38)
ADVERSE REACTIONS
Adverse reactions to 17D yellow fever vaccine include mild headaches, myalgia, low-grade fevers, or other minor symptoms for 5 to 10 days. Local reactions including edema, hypersensitivity, pain or mass at the injection site have also been reported following yellow fever vaccine administration. Immediate hypersensitivity reactions, characterized by rash, urticaria, and/or asthma, are uncommon and occur principally among persons with histories of egg allergy. (1) (2) (24)
No placebo-controlled trials to assess the safety of yellow fever 17D vaccines have been performed. However, between 1953 and 1994, reactogenicity of 17D-204 vaccine was monitored in 10 uncontrolled clinical trials. The trials included a total of 3,933 adults and 264 infants greater than 4 months old residing in Europe or in yellow fever endemic areas. Self-limited and mild local reactions consisting of erythema and pain at the injection site and systemic reactions consisting of headache and/or fever occurred in a minority of subjects (typically less than 5%) 5 to 7 days after immunization. In one study involving 115 infants age 4 to 24 months the incidence of fever was as high as 21%. Also in this study, reactogenicity of the vaccine was markedly reduced among a subset of subjects who had serological evidence of previous exposure to yellow fever virus. Only two of the ten studies provided diary cards for daily reporting; this method resulted in a slightly higher incidence of local and systemic complaints. (1)
In 2001, YF-VAX vaccine was used as a control in a double-blind, randomized comparative trial with another 17D-204 vaccine, conducted at nine centers in the US. YF-VAX vaccine was administered to 725 adults ≥18 years old with a mean age of 38 years. Safety data were collected by diary card for days 1 through 10 after vaccination and by interview on days 5, 11, and 31. Among subjects who received YF-VAX vaccine, there were no serious adverse events, and 71.9% experienced non-serious adverse events judged to have been related to vaccination. Most of these were injection site reactions of mild to moderate severity. Four such local reactions were considered severe. Rash occurred in 3.2% and urticaria in two subjects. Systemic reactions (headache, myalgia, malaise, and asthenia) were usually mild and occurred in 10% to 30% of subjects during the first few days after vaccination. The incidence of non-serious adverse reactions, including headache, malaise, injection site edema, and pain, was significantly lower in subjects >60 years compared to younger subjects. Adverse events were less frequent in the 1.7% of vaccinated subjects who had pre-existing immunity to yellow fever virus, compared to those who had not been previously exposed. (16)
A CDC analysis of data submitted to the Vaccine Adverse Events Reporting System (VAERS) between 1990 and 1998 suggests that patients aged 65 or older are at increased risk for systemic adverse events temporally associated with vaccination, compared to the 25- to 44-year-old age group (see PRECAUTIONS section, Geriatric Use subsection). The rate of systemic adverse events occurring post-vaccination in patients age 65 to 74 was 2.5 times higher than the rate occurring in patients age 25 to 44, based on incidence rates of 6.21 and 2.49 per 100,000 doses of vaccine in the two groups, respectively. (38)
Neurotropic Disease
Vaccine-associated neurotropic disease (2), previously described as post-vaccinal encephalitis (1), is a known rare serious adverse event associated with 17D vaccination. Age less than 9 months and immunosuppression are known risk factors. Twenty-one cases of vaccine-associated neurotropic disease associated with all licensed 17D vaccines have been reported between 1952 and 2004, 18 in children or adolescents. Fifteen of these cases occurred prior to 1960, thirteen of which occurred in infants 4 months of age or younger, and two of which occurred in infants six and seven months old. Six cases were reported between 1960 and 1996, world-wide. Three occurred in children, including a one-month-old infant, a three-year-old, and a thirteen-year-old. The three-year-old died of encephalitis, and a genetic variant of the vaccine virus was isolated from the brain in this case. (39) This is the only verified fatality due to yellow fever vaccine-associated neurotropic disease. The three remaining cases of vaccine-associated neurotropic disease since 1960 occurred in adults. (1)
The incidence of vaccine-associated neurotropic disease in infants less than 4 months old is estimated to be between 0.5 and 4 per 1,000, based on two historical reports where denominators are available. (40) (41) No data are available for calculation of an age-specific incidence rate in the 4- to 9-month-age group. A study in Senegal (42) described two fatal cases of encephalitis possibly associated with 17D-204 vaccination among 67,325 children between the ages of 6 months and 2 years, for an incidence rate of 3 per 100,000. One study conducted in Kenya in 1993 detected four cases of encephalitis temporally associated with vaccination, one in a 2-year-old child and three in adults, for an incidence of 5.3 cases per million vaccinees of all ages. (1)
Other very rare neurological signs and symptoms have been reported and include Guillain-Barré syndrome, seizures and focal neurological deficits. (43)
Viscerotropic Disease
Vaccine-associated viscerotropic disease, previously described as multiple organ system failure (26), is a known rare serious adverse event associated with 17D vaccination. No cause and effect relationship has been established between vaccination and these subsequent illnesses. Physicians should therefore be cautious to administer yellow fever vaccine only to those persons truly at risk of exposure to wild-type yellow fever virus infection. (2)
Between 1996 and 1998, four patients, ages 63, 67, 76, and 79, became severely ill 2 to 5 days after vaccination with YF-VAX vaccine. Three of these 4 subjects died. The clinical presentations were characterized by a non-specific febrile syndrome with fatigue, myalgia, and headache, rapidly progressing to a severe illness including respiratory failure, elevated hepatocellular enzymes, lymphocytopenia and thrombocytopenia, hyperbilirubinemia, and renal failure requiring hemodialysis. (26) None of these subjects had vaccine-associated neurotropic disease. In two cases where vaccine virus was recovered from serum, limited nucleotide sequence analysis of the viral genome suggested that the isolates had not undergone a mutation associated with an increase in virulence. The incidence rate for these serious adverse events was estimated at 1 per 400,000 doses of YF-VAX vaccine, based on the total number of doses administered in the US civilian population during the surveillance period.
Vaccine-associated viscerotropic disease temporally associated with yellow fever vaccination has also been reported in Australia and Brazil. One Australian citizen became ill after receiving an immunization with the 17D-204 strain of yellow fever vaccine in his home country, (28) and two Brazilian citizens (age 5 and 22 years) became ill three to four days after receiving 17DD vaccine in Brazil. (29) In the Brazilian and Australian cases, histopathologic changes in the liver included midzonal necrosis, microvesicular fatty change, and Councilman bodies, which are characteristic of wild-type yellow fever. Vaccine-type yellow fever virus was isolated from blood and autopsy material (ie, brain, liver, kidney, spleen, lung, skeletal muscle, or skin) of each of these three persons, all of whom died 8 to 11 days after vaccination. In Brazil, an estimated 23 million vaccine doses were administered during the 15-month period during which the two cases of multiple organ system failure were reported. (29)
In view of the data cited above, both the 17D-204 and 17DD yellow fever vaccines may be considered as a possible, but rare, cause of vaccine-associated viscerotropic disease (2) that is similar to fulminant yellow fever caused by wild-type yellow fever virus. All available evidence from complete nucleotide sequence analysis and testing in experimental animals of vaccine-type yellow fever viruses isolated from the Brazilian subjects suggests that the occurrences are due to undefined host factors, rather than to intrinsic virulence of the 17DD vaccine viruses. (27)
Because of a lack of tissue specimens from most of the US cases of vaccine-associated viscerotropic disease and the qualitative differences between the US cases and those identified in Brazil and Australia, no definitive support for a causal relationship exists between receipt of YF-VAX vaccine and vaccine-associated viscerotropic disease. However, the temporal association with recent receipt of yellow fever vaccine and the similarity of the clinical presentations among all four US cases suggest that the vaccine may play a role in pathogenesis of the cases.
Pregnancy
Safety of YF-VAX vaccine was evaluated in a study involving 101 Nigerian women, the majority of whom (88%) were in the third trimester of pregnancy. In this study, it appeared that vaccinating pregnant women with the 17D-204 strain of yellow fever vaccine was not associated with adverse events affecting the mother or fetus. There were no adverse events among 40 infants who were carefully followed up for one year after birth, and none of these infants tested positive for IgM antibodies as a criterion for transplacental infection. However, the percentage of pregnant women who seroconverted was significantly reduced compared to a non-pregnant control group (38.6% vs. 81.5%). (18)
Following a mass immunization campaign in Trinidad, during which 100 to 200 pregnant females were immunized, no adverse events related to pregnancy were reported. In addition, 41 cord blood samples were obtained from infants born to mothers immunized during the first trimester. One of these infants tested positive for IgM antibodies in cord blood. The infant appeared normal at delivery and no subsequent adverse sequelae of infection were reported. However, this result suggests that transplacental infection with 17D vaccine viruses can occur. (44)
A recent case-control study of spontaneous abortion following vaccination of Brazilian women found no significant difference in the odds ratio among vaccinated women compared to a similar unvaccinated group. (45)
Reporting of Adverse Events
The US Department of Health and Human Services has established the Vaccine Adverse Event Reporting System (VAERS) to accept all reports of suspected adverse events after the administration of any vaccine. Reporting of all adverse events occurring after vaccine administration is encouraged from vaccine recipients, parents/guardians and the health care provider. Adverse events following immunization should be reported to VAERS. Reporting forms and information about reporting requirements or completion of the form can be obtained from VAERS through a toll-free number 1-800-822-7967. (30) Reporting forms may also be obtained at the FDA web site at http://vaers.hhs.gov.
Health-care providers also should report these events to Sanofi Pasteur Inc., Discovery Drive, Swiftwater, PA 18370 or call 1-800-822-2463.
DOSAGE AND ADMINISTRATION
Primary Vaccination: For all eligible persons, a single subcutaneous injection of 0.5 mL of reconstituted vaccine (formulated to contain not less than 4.74 log10 PFU throughout the life of the product) should be administered. Immunity develops by the 10th day after primary vaccination. (11) (25) (46)
Booster Doses: Re-immunization with 17D vaccine is recommended every 10 years for those at continuing risk of exposure and is required by International Health Regulations. (23) Revaccination boosts antibody titer, although evidence from several studies suggests that yellow fever vaccine immunity persists for at least 30 to 35 years and probably for life, (47) and epidemiologic data suggest that a single infection with wild-type yellow fever virus provides lifelong immunity against illness due to subsequent exposure.
Concomitant Administration with other Vaccines
Determination of whether to administer yellow fever vaccine and other immunobiologics simultaneously should be made on the basis of convenience to the traveler in completing the desired vaccinations before travel and on information regarding possible interference. Limited data are available related to administration of YF-VAX vaccine with other vaccines. (See PRECAUTIONS section, Drug Interactions subsection.) In those specific instances where vaccines may be given concurrently, injections should be administered at separate sites. Where there are no data to support administration of YF-VAX vaccine concurrently with other vaccines, 4 weeks should elapse between sequential vaccinations. (2)
Vaccine Preparation
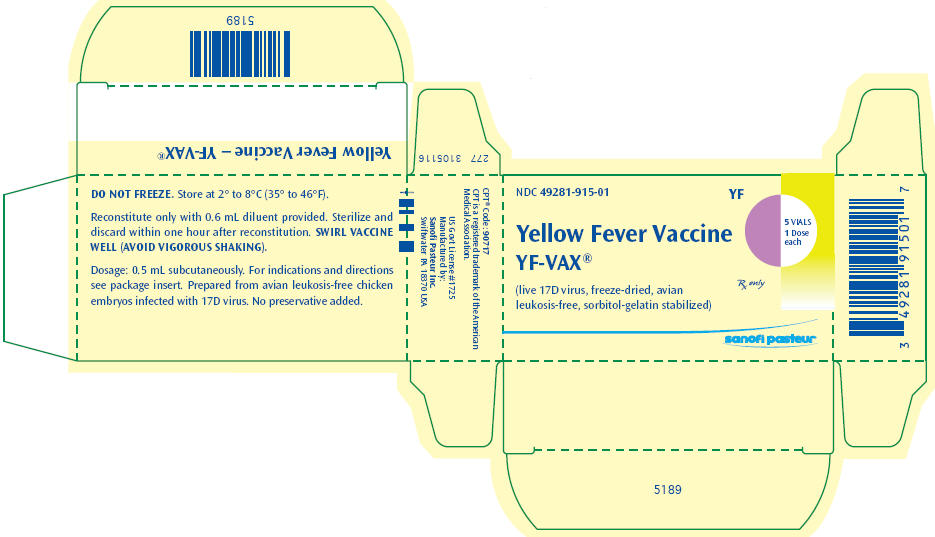
- Reconstitute the vaccine using only the diluent supplied (0.6 mL vial of Sodium Chloride Injection USP for single dose vial of vaccine and 3 mL vial of Sodium Chloride Injection USP for 5 dose vial of vaccine). Draw the volume of the diluent, shown on the diluent label, into a suitable size syringe and slowly inject into the vial containing the vaccine. Allow the reconstituted vaccine to sit for one to two minutes and then carefully swirl mixture until a uniform suspension is achieved. Avoid vigorous shaking as this tends to cause foaming of the suspension. Do not dilute reconstituted vaccine.
- YF-VAX vaccine is a slight pink-brown suspension after reconstitution. If the product contains extraneous particulate matter or is discolored, do not administer the vaccine.
- SWIRL VACCINE WELL before withdrawing each dose. Administer the single immunizing dose of 0.5 mL subcutaneously using a 5/8- to 3/4-inch long needle (23) within 60 minutes of reconstituting the vial.
Properly dispose of all reconstituted vaccine and containers that remain unused after one hour (eg, sterilized or disposed in red hazardous waste containers). (2)
Desensitization
(23)
If immunization is imperative and the individual has a history of severe egg sensitivity and has a positive skin test to the vaccine, this desensitization procedure may be used to administer the vaccine.
The following successive doses should be administered subcutaneously at 15- to 20-minute intervals:
- 0.05 mL of 1:10 dilution
- 0.05 mL of full strength
- 0.10 mL of full strength
- 0.15 mL of full strength
- 0.20 mL of full strength
Desensitization should only be performed under the direct supervision of a physician experienced in the management of anaphylaxis with necessary emergency equipment immediately available.
HOW SUPPLIED
Vial, 1 Dose (5 per package) with 0.6 mL vial of diluent (5 per package) for administration with needle and syringe. Product No. 49281-915-01
Vial, 5 Dose with 3 mL vial of diluent, for administration with needle and syringe. Product No. 49281-915-05
| YF-VAX vaccine (Yellow Fever Vaccine) in the US is supplied only to designated Yellow Fever Vaccination Centers authorized to issue valid certificates of Yellow Fever Vaccination. Location of the nearest Yellow Fever Vaccination Centers may be obtained from the Centers for Disease Control and Prevention, Atlanta, GA 30333, state or local health departments. |
STORAGE
Store at 2° to 8°C (35° to 46°F). DO NOT FREEZE.
Do not use vaccine after expiration date. YF-VAX vaccine does not contain a preservative; therefore, all reconstituted vaccine and containers, which remain unused after one hour must be properly disposed (eg, sterilized or disposed in red hazardous waste containers). (2)
The following stability information for YF-VAX vaccine is provided for those countries or areas of the world where an adequate cold chain is a problem and inadvertent exposure to abnormal temperatures has occurred. Half-life is reduced from approximately 14 days at 35° to 37°C to 3-4 days at 45° to 47°C.
YF-VAX vaccine is formulated to satisfy the current US potency requirements of not less than 4.74 log10 PFU per 0.5 mL dose throughout the life of the product and meets the minimum requirements of WHO. (10)
REFERENCES
- 1
- Monath TP. Yellow Fever. Plotkin SA, Orenstein WA (eds.). Vaccines. 3rd Edition, WB Saunders Company. 1999;815-879.
- 2
- Recommendations of the Advisory Committee on Immunization Practices (ACIP). Yellow Fever Vaccine. 2002. MMWR 2002;51(RR17):1-10.
- 3
- Teichmann D, et al. A haemorrhagic fever from the Côte d'Ivoire. 1999. Lancet 354:1608.
- 4
- ACIP. Fatal yellow fever in a traveler returning from Venezuela, 1999. MMWR 2000;49(14):303-305.
- 5
- McFarland JM, et al. Imported yellow fever in a United States citizen. Clin Infect Dis 1997;25:1143-1147.
- 6
- Centers for Disease Control and Prevention (CDC) Fatal yellow fever in a traveler returning from the Amazonas, Brazil, 2002. MMWR 2002;51(15):324-325.
- 7
- World Health Organization (WHO). Imported case of yellow fever, Belgium. Weekly Epidemiological Record 2001;76:357,365.
- 8
- Barros MLB, Boecken G. Jungle yellow fever in the central Amazon. Lancet 1996;348:969-970.
- 9
- Mason RA, et al. Yellow fever vaccine: Direct challenge of monkeys given graded doses of 17D vaccine. Appl Microbiol 1973;25(4):539-544.
- 10
- Requirements for yellow fever vaccine. WHO Technical Report Series. 1976;594:23-49.
- 11
- Wisseman CL, et al. Immunological studies with Group B arthropod-borne viruses. Am J Trop Med Hyg 1962;11:550-561.
- 12
- Dukes C, et al. Safety and Immunogenicity of Simultaneous Administration of Typhim Vi (TV), YF-VAX (YV), and Menomune (MV). [abstract]. American Society for Microbiology. The 36th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC): 1996; September 15-18:159.
- 13
- Meyer HM, et al. Response of Volta children to jet inoculation of combined live measles, smallpox, and yellow fever vaccines. Bull World Health Org 1964;30:783-794.
- 14
- Bancroft WH, et al. Dengue virus type 2 vaccine: reactogenicity and immunogenicity in soldiers. J Infect Dis 1984;149:1005-1010.
- 15
- Jackson J, et al. Comparison of Antibody Response and Patient Tolerance of Yellow Fever Vaccine Administered by the Bioject Needle-Free Injection System versus Conventional Needle/Syringe Injection. Third International Conference on Travel Medicine; Paris 1993;April:25-29;264:209.
- 16
- Monath TP, et al. Comparative safety and immunogenicity of two yellow fever 17D vaccines (ARILVAX and YF-VAX) in a Phase III multicenter, double-blind clinical trial. Am J Trop Med Hyg 66(5)2002;533-541.
- 17
- Goujon C, et al. Good Tolerance and Efficacy of Yellow Fever Vaccine Among Subjects Carriers of Human Immunodeficiency Virus. Fourth International Conference on Travel Medicine; Acapulco, Mexico 1995; April:23-27;32:63.
- 18
- Nasidi A, et al. Yellow fever vaccination and pregnancy: a four-year prospective study. Transactions of the Royal Society of Tropical Medicine and Hygiene 1993;87:337-339.
- 19
- Bonnevie-Nielson V, et al. Lymphocytic 2',5' - Oligoadenylate synthetase activity increases prior to the appearance of neutralizing antibodies and Immunoglobulin M and Immunoglobulin G antibodies after primary and secondary immunization with yellow fever vaccine. Clin Diag Lab Immunol 1995;2:302-306.
- 20
- Smithburn KC, et al. Immunization against yellow fever: Studies on the time of development and the duration of induced immunity. Am J Trop Med Page 7 of 8 Hyg 1945;45:217-223.
- 21
- World Health Organization (WHO). International Health Regulations (1969) (3rd annotated edition). Geneva 1983:30-65.
- 22
- CDC. Vaccine Information Statements (VIS) - Yellow Fever Vaccine [serial online]. Available at:http://www.cdc.gov/vaccines/pubs/vis/vis-yf.pdf. Accessed August 9, 2007.
- 23
- American Academy of Pediatrics. In:Pickering LK, ed. 2000 Red Book: Report of the Committee on Infectious Diseases. 25th ed. Elk Grove Village, IL: American Academy of Pediatrics 2000;35-38,174-175.
- 24
- Sanofi Pasteur Inc. Data on File – 080601;120104.
- 25
- CDC. Health Information for the International Traveler, 2001-2002. Atlanta: US Department of Health and Human Services, Public Health Service 2001;3- 6,12-21,154-160,207-220.
- 26
- Martin M, et al. Fever and multisystem organ failure associated with 17D-204 yellow fever vaccination: a report of four cases. Lancet 2001;358:98-104.
- 27
- Galler R, et al. Phenotypic and molecular analyses of yellow fever 17DD vaccine viruses associated with serious adverse events in Brazil. Virology 2001;290:309-319.
- 28
- Chan RC, et al. Hepatitis and death following vaccination with yellow fever 17D-204 vaccine. Lancet 2001;358:121-122.
- 29
- Vasconcelos PFC, et al. Serious adverse events associated with yellow fever 17DD vaccine in Brazil: a report of two cases. Lancet 2001;358:91-97.
- 30
- CDC. Vaccine Adverse Event Reporting System - United States. MMWR 1990;39:730-733.
- 31
- CDC. National Childhood Vaccine Injury Act: Requirements for Permanent Vaccination Records and for Reporting of Selected Events after Vaccination. MMWR 1988;37(13):197-200.
- 32
- Food and Drug Administration. New reporting requirements for vaccine adverse events. FDA Drug Bull 1988;18(2):16-18.
- 33
- Ruben FL, et al. Simultaneous administration of smallpox, measles, yellow fever, and diphtheria-pertussis-tetanus antigens to Nigerian children. Bull WHO 1973;48:175-181.
- 34
- Dumas R, et al. Safety and immunogenicity of a new inactivated hepatitis A vaccine and concurrent administration with a typhoid fever vaccine or a typhoid fever + yellow fever vaccine. Adv Therapy 1997;14:160-167.
- 35
- Coursaget P, et al. Simultaneous injection of plasma-derived or recombinant hepatitis B vaccines with yellow fever and killed polio vaccines. Vaccine 1995;13:109-111.
- 36
- Kaplan JE, et al. The effect of immune globulin on the response to trivalent oral poliovirus and yellow fever vaccinations. Bull WHO 1984;62(4):585-590.
- 37
- Tsai TF, et al. Chloroquine does not adversely affect the antibody response to yellow fever vaccine. J Infect Dis 1986;154(4):726-727.
- 38
- Martin M, et al. Advanced age a risk factor for illness temporally associated with yellow fever vaccination. Emerg Infect Dis 2001;7:945-951.
- 39
- Jennings AD, et al. Analysis of a yellow fever virus isolated from a fatal case of vaccine-associated human encephalitis. J Infect Dis 1994;169:512-518.
- 40
- Stuart G. Reactions following vaccination against yellow fever. In Smithburn KC, Durieux C, Koerber R, et al (eds.). Yellow Fever Vaccination. Geneva, WHO 1956;143-189.
- 41
- Louis JJ, et al. A case of encephalitis after 17D strain yellow fever vaccination. Pediatr 1981;36(7):547-550.
- 42
- Rey M, et al. Epidemiological and clinical aspects of encephalitis following yellow fever vaccination. Bull Soc Méd Afr Noire Lgue fr 1966;v XI,(3),560-574.
- 43
- Sanofi Pasteur Inc. Data on File - 080207.
- 44
- Tsai TF, et al. Congenital yellow fever virus infection after immunization in pregnancy. J Infect Dis 1993;168:1520-1523.
- 45
- Nishioka SA, et al. Yellow fever vaccination during pregnancy and spontaneous abortion: a case-control study. Trop Med Int Health 1998;3(1):29-33.
- 46
- ACIP. General Recommendations on Immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP). MMWR 2002;51(RR02):1-35.
- 47
- Poland JD, et al. Persistence of neutralizing antibody 30-35 years after immunization with 17D yellow fever vaccine. Bull WHO 1981;59(6):895-900.
Product Information as of January 2010
Manufactured by:
Sanofi Pasteur Inc.
Swiftwater PA 18370 USA
5802-5803
277 3112544/3112545
PRINCIPAL DISPLAY PANEL - Vial Label
NDC 49281-915-01
Yellow Fever
Vaccine
YF-VAX®
YF
1 Dose
(0.5 mL)
Rx only
Mdf by: Sanofi Pasteur Inc.

PRINCIPAL DISPLAY PANEL - Vial Carton
NDC 49281-915-01
YF
Yellow Fever Vaccine
YF-VAX®
(live 17D virus, freeze-dried, avian
leukosis-free, sorbitol-gelatin stabilized)
5 VIALS
1 Dose
each
Rx only
sanofi pasteur
| YF-VAX
yellow fever virus live antigen, a kit |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA103915 | 05/22/1953 | |
| Labeler - Sanofi Pasteur Inc. (086723285) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Sanofi Pasteur Inc. | 086723285 | MANUFACTURE | |