cubicin (Daptomycin) injection, powder, lyophilized, for solution
[Integrated Commercialization Solutions]
SPL UNCLASSIFIED SECTION
To reduce the development of drug-resistant bacteria and maintain the effectiveness of CUBICIN and other antibacterial drugs, CUBICIN should be used only to treat or prevent infections caused by bacteria.
DESCRIPTION
CUBICIN contains daptomycin, a cyclic lipopeptide antibacterial agent derived from the fermentation of Streptomyces roseosporus. The chemical name is N-decanoyl-L-tryptophyl-D-asparaginyl-L-aspartyl-L-threonylglycyl-L-ornithyl-L-aspartyl-D-alanyl-L-aspartylglycyl-D-seryl-threo-3-methyl-L-glutamyl-3-anthraniloyl-L-alanine ε1-lactone. The chemical structure is:
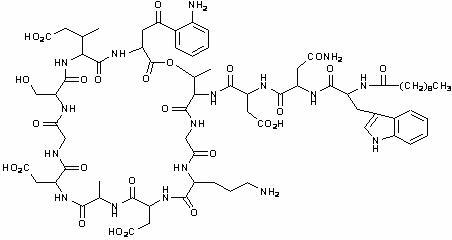
The empirical formula is C72H101N17O26; the molecular weight is 1620.67. CUBICIN is supplied as a sterile, preservative-free, pale yellow to light brown, lyophilized cake containing approximately 900 mg/g of daptomycin for intravenous (IV) use following reconstitution with 0.9% sodium chloride injection. The only inactive ingredient is sodium hydroxide, which is used in minimal quantities for pH adjustment. Freshly reconstituted solutions of CUBICIN range in color from pale yellow to light brown.
CLINICAL PHARMACOLOGY
Pharmacokinetics
The mean (SD) pharmacokinetic parameters of daptomycin at steady-state following IV administration of 4 to 12 mg/kg q24h to healthy young adults are summarized in Table 1.
Daptomycin pharmacokinetics were generally linear and time-independent at doses of 4 to 12 mg/kg q24h. Steady-state trough concentrations were achieved by the third daily dose. The mean (SD) steady-state trough concentrations attained following administration of 4, 6, 8, 10, and 12 mg/kg q24h were 5.9 (1.6), 6.7 (1.6), 10.3 (5.5), 12.9 (2.9), and 13.7 (5.2) µg/mL, respectively.
| Pharmacokinetic Parametersa | |||||
| Doseb | AUC0-24 | t1/2 | Vss | CLT | Cmax |
| (mg/kg) | (µg*h/mL) | (h) | (L/kg) | (mL/h/kg) | (µg/mL) |
| a. AUC0-24, area under the concentration-time curve from 0 to 24 hours; t½, terminal elimination half-life; Vss, volume of distribution at steady-state; CLT, plasma clearance; Cmax, maximum plasma concentration. | |||||
| b. Doses of CUBICIN in excess of 6 mg/kg have not been approved. | |||||
| 4 (N=6) | 494 (75) | 8.1 (1.0) | 0.096 (0.009) | 8.3 (1.3) | 57.8 (3.0) |
| 6 (N=6) | 632 (78) | 7.9 (1.0) | 0.101 (0.007) | 9.1 (1.5) | 93.9 (6.0) |
| 8 (N=6) | 858 (213) | 8.3 (2.2) | 0.101 (0.013) | 9.0 (3.0) | 123.3 (16.0) |
| 10 (N=9) | 1039 (178) | 7.9 (0.6) | 0.098 (0.017) | 8.8 (2.2) | 141.1 (24.0) |
| 12 (N=9) | 1277 (253) | 7.7 (1.1) | 0.097 (0.018) | 9.0 (2.8) | 183.7 (25.0) |
Distribution
Daptomycin is reversibly bound to human plasma proteins, primarily to serum albumin, in a concentration-independent manner. The overall mean binding ranged from 90 to 93%.
In clinical studies, mean serum protein binding in subjects with CLCR≥30 mL/min was comparable to that observed in healthy subjects with normal renal function. However, there was a trend toward decreasing serum protein binding among subjects with CLCR<30 mL/min (87.6%), including those receiving hemodialysis (85.9%) and continuous ambulatory peritoneal dialysis (CAPD) (83.5%). The protein binding of daptomycin in subjects with hepatic impairment (Child-Pugh B) was similar to that in healthy adult subjects.
The volume of distribution at steady-state (Vss) of daptomycin in healthy adult subjects was approximately 0.10 L/kg and was independent of dose.
Metabolism
In vitro studies with human hepatocytes indicate that daptomycin does not inhibit or induce the activities of the following human cytochrome P450 isoforms: 1A2, 2A6, 2C9, 2C19, 2D6, 2E1, and 3A4. In in vitro studies, daptomycin was not metabolized by human liver microsomes. It is unlikely that daptomycin will inhibit or induce the metabolism of drugs metabolized by the P450 system.
In 5 healthy young adults after infusion of radiolabeled 14C-daptomycin, the plasma total radioactivity was similar to the concentration determined by microbiological assay. In a separate study, no metabolites were observed in plasma on Day 1 following administration of CUBICIN at 6 mg/kg to subjects. Inactive metabolites have been detected in urine, as determined by the difference in total radioactive concentrations and microbiologically active concentrations. Minor amounts of three oxidative metabolites and one unidentified compound were detected in urine. The site of metabolism has not been identified.
Excretion
Daptomycin is excreted primarily by the kidney. In a mass balance study of 5 healthy subjects using radiolabeled daptomycin, approximately 78% of the administered dose was recovered from urine based on total radioactivity (approximately 52% of the dose based on microbiologically active concentrations) and 5.7% of the dose was recovered from feces (collected for up to 9 days) based on total radioactivity.
Because renal excretion is the primary route of elimination, dosage adjustment is necessary in patients with severe renal insufficiency (CLCR<30 mL/min) (see DOSAGE AND ADMINISTRATION).
Special Populations
Renal Insufficiency
Population derived pharmacokinetic parameters were determined for infected patients (complicated skin and skin structure infections and S. aureus bacteremia) and noninfected subjects with varying degrees of renal function (Table 2). Plasma clearance (CLT), elimination half-life (t1/2), and volume of distribution (Vss) were similar in patients with complicated skin and skin structure infections compared with those with S. aureus bacteremia. Following the administration of CUBICIN 4 mg/kg q24h, the mean CLT was 9%, 22%, and 46% lower among subjects and patients with mild (CLCR 50-80 mL/min), moderate (CLCR 30-50 mL/min), and severe (CLCR<30 mL/min) renal impairment, respectively, than in those with normal renal function (CLCR>80 mL/min). The mean steady-state systemic exposure (AUC), t1/2, and Vss increased with decreasing renal function, although the mean AUC was not markedly different for patients with CLCR 30-80 mL/min compared with those with normal renal function. The mean AUC for patients with CLCR<30 mL/min and for patients on hemodialysis (dosed post-dialysis) was approximately 2 and 3 times higher, respectively, than for patients with normal renal function. Following the administration of CUBICIN 4 mg/kg q24h, the mean Cmax ranged from 60 to 70 µg/mL in patients with CLCR≥30 mL/min, while the mean Cmax for patients with CLCR<30 mL/min ranged from 41 to 58 µg/mL. The mean Cmax ranged from 80 to 114 µg/mL in patients with mild-to-moderate renal impairment and was similar to that of patients with normal renal function after the administration of CUBICIN 6 mg/kg q24h. In patients with renal insufficiency, both renal function and creatine phosphokinase (CPK) should be monitored more frequently. CUBICIN should be administered following the completion of hemodialysis on hemodialysis days (see DOSAGE AND ADMINISTRATION for recommended dosage regimens).
| t1/2a | Vssa | CLTa | AUC0-∞a | AUCssb | Cmin,ssb | |
| Renal Function | (h) | (L/kg) | (mL/h/kg) | (µg*h/mL) | (µg*h/mL) | (µg*h/mL) |
| 4 mg/kg | 4 mg/kg | 4 mg/kg | 4 mg/kg | 6 mg/kg | 6 mg/kg | |
| Note: CLCR, creatinine clearance estimated using the Cockcroft-Gault equation with actual body weight; AUC0-∞, area under the concentration-time curve extrapolated to infinity; AUCss, area under the concentration-time curve calculated over the 24-hour dosing interval at steady-state; Cmin,ss, trough concentration at steady-state; NA, not applicable. | ||||||
| a. Parameters obtained following a single dose from patients with complicated skin and skin structure infections and healthy subjects. | ||||||
| b. Parameters obtained at steady-state from patients with S. aureus bacteremia. | ||||||
| Normal | 9.39 (4.74) | 0.13 (0.05) | 10.9 (4.0) | 417 (155) | 545 (296) | 6.9 (3.5) |
| (CLCR>80 mL/min) | N=165 | N=165 | N=165 | N=165 | N=62 | N= 61 |
| Mild Renal Impairment | 10.75 (8.36) | 0.12 (0.05) | 9.9 (4.0) | 466 (177) | 637 (215) | 12.4 (5.6) |
| (CLCR 50-80 mL/min) | N=64 | N=64 | N=64 | N=64 | N=29 | N=29 |
| Moderate Renal Impairment | 14.70 (10.50) | 0.15 (0.06) | 8.5 (3.4) | 560 (258) | 868 (349) | 19.0 (9.0) |
| (CLCR 30-<50 mL/min) | N=24 | N=24 | N=24 | N=24 | N=15 | N=14 |
| Severe Renal Impairment | 27.83 (14.85) | 0.20 (0.15) | 5.9 (3.9) | 925 (467) | 1050, 892 | 24.4, 21.4 |
| (CLCR<30 mL/min) | N=8 | N=8 | N=8 | N=8 | N=2 | N=2 |
| Hemodialysis | 29.81 (6.13) | 0.15 (0.04) | 3.7 (1.9) | 1244 (374) | NA | NA |
| N=21 | N=21 | N=21 | N=21 | |||
Hepatic Insufficiency
The pharmacokinetics of daptomycin were evaluated in 10 subjects with moderate hepatic impairment (Child-Pugh Class B) and compared with healthy volunteers (N=9) matched for gender, age, and weight. The pharmacokinetics of daptomycin were not altered in subjects with moderate hepatic impairment. No dosage adjustment is warranted when administering CUBICIN to patients with mild-to-moderate hepatic impairment. The pharmacokinetics of daptomycin in patients with severe hepatic insufficiency have not been evaluated.
Gender
No clinically significant gender-related differences in daptomycin pharmacokinetics have been observed. No dosage adjustment is warranted based on gender when administering CUBICIN.
Geriatric
The pharmacokinetics of daptomycin were evaluated in 12 healthy elderly subjects (≥75 years of age) and 11 healthy young controls (18 to 30 years of age). Following administration of a single 4 mg/kg IV dose, the mean total clearance of daptomycin was reduced approximately 35% and the mean AUC0-∞ increased approximately 58% in elderly subjects compared with young healthy subjects. There were no differences in Cmax. No dosage adjustment is warranted for elderly patients with normal renal function.
Obesity
The pharmacokinetics of daptomycin were evaluated in 6 moderately obese (Body Mass Index [BMI] 25 to 39.9 kg/m2) and 6 extremely obese (BMI ≥40 kg/m2) subjects and controls matched for age, sex, and renal function. Following administration of a single 4 mg/kg IV dose based on total body weight, the plasma clearance of daptomycin normalized to total body weight was approximately 15% lower in moderately obese subjects and 23% lower in extremely obese subjects compared with nonobese controls. The AUC0-∞ of daptomycin increased approximately 30% in moderately obese and 31% in extremely obese subjects compared with nonobese controls. The differences were most likely due to differences in the renal clearance of daptomycin. No dosage adjustment of CUBICIN is warranted in obese subjects.
Pediatric
The pharmacokinetics of daptomycin in pediatric populations (<18 years of age) have not been established.
Drug-Drug Interactions
Drug-drug interaction studies were performed with CUBICIN and other drugs that are likely to be either co-administered or associated with overlapping toxicity.
Aztreonam
In a study in which 15 healthy adult subjects received a single dose of CUBICIN 6 mg/kg IV, aztreonam 1 g IV, and both in combination, the Cmax and AUC0-∞ of daptomycin were not significantly altered by aztreonam; the Cmax and AUC0-∞ of aztreonam also were not significantly altered by daptomycin. No dosage adjustment of either antibiotic is warranted when co-administered.
Tobramycin
In a study in which 6 healthy adult males received a single dose of CUBICIN 2 mg/kg IV, tobramycin 1 mg/kg IV, and both in combination, the mean Cmax and AUC0-∞ of daptomycin increased 12.7% and 8.7%, respectively, when administered with tobramycin. The mean Cmax and AUC0-∞ of tobramycin decreased 10.7% and 6.6%, respectively, when administered with CUBICIN. These differences were not statistically significant. The interaction between daptomycin and tobramycin with a clinical dose of CUBICIN is unknown. Caution is warranted when CUBICIN is co-administered with tobramycin.
Warfarin
In 16 healthy subjects, concomitant administration of CUBICIN 6 mg/kg q24h for 5 days followed by a single oral dose of warfarin (25 mg) had no significant effect on the pharmacokinetics of either drug and did not significantly alter the INR (International Normalized Ratio) (see PRECAUTIONS, Drug Interactions).
Simvastatin
In 20 healthy subjects on a stable daily dose of simvastatin 40 mg, administration of CUBICIN 4 mg/kg IV q24h for 14 days (N=10) was not associated with a higher incidence of adverse events than in subjects receiving placebo once daily (N=10) (see PRECAUTIONS, Drug Interactions).
Probenecid
Concomitant administration of probenecid (500 mg 4 times daily) and a single dose of CUBICIN 4 mg/kg IV did not significantly alter the Cmax and AUC0-∞ of daptomycin. No dosage adjustment of CUBICIN is warranted when CUBICIN is co-administered with probenecid.
MICROBIOLOGY
Daptomycin is an antibacterial agent of a new class of antibiotics, the cyclic lipopeptides. Daptomycin is a natural product that has clinical utility in the treatment of infections caused by aerobic Gram-positive bacteria. The in vitro spectrum of activity of daptomycin encompasses most clinically relevant Gram-positive pathogenic bacteria. Daptomycin retains potency against antibiotic-resistant Gram-positive bacteria, including isolates resistant to methicillin, vancomycin, and linezolid.
Daptomycin exhibits rapid, concentration-dependent bactericidal activity against Gram-positive organisms in vitro. This has been demonstrated both by time-kill curves and by MBC/MIC ratios (minimum bactericidal concentration/minimum inhibitory concentration) using broth dilution methodology. Daptomycin maintained bactericidal activity in vitro against stationary phase S. aureus in simulated endocardial vegetations. The clinical significance of this is not known.
Mechanism of Action
The mechanism of action of daptomycin is distinct from that of any other antibiotic. Daptomycin binds to bacterial membranes and causes a rapid depolarization of membrane potential. This loss of membrane potential causes inhibition of protein, DNA, and RNA synthesis, which results in bacterial cell death.
Mechanism of Resistance
At this time, no mechanism of resistance to daptomycin has been identified. Currently, there are no known transferable elements that confer resistance to daptomycin.
Cross-Resistance
Cross-resistance has not been observed with any other antibiotic class.
Interactions with Other Antibiotics
In vitro studies have investigated daptomycin interactions with other antibiotics. Antagonism, as determined by kill curve studies, has not been observed. In vitro synergistic interactions of daptomycin with aminoglycosides, β-lactam antibiotics, and rifampin have been shown against some isolates of staphylococci (including some methicillin-resistant isolates) and enterococci (including some vancomycin-resistant isolates).
Complicated Skin and Skin Structure Infection (cSSSI) Studies
The emergence of daptomycin non-susceptible isolates occurred in 2 infected patients across the set of Phase 2 and pivotal Phase 3 clinical trials. In one case, a non-susceptible S. aureus was isolated from a patient in a Phase 2 study who received CUBICIN at a less than the protocol-specified dose for the initial 5 days of therapy. In the second case, a non-susceptible Enterococcus faecalis was isolated from a patient with an infected chronic decubitus ulcer enrolled in a salvage trial.
S. aureus Bacteremia/Endocarditis and Other Post-Approval Studies
In subsequent clinical trials, non-susceptible isolates were recovered. S. aureus was isolated from a patient in a compassionate-use study and from 7 patients in the S. aureus bacteremia/endocarditis study (see PRECAUTIONS). An E. faecium was isolated from a patient in a VRE study.
Daptomycin has been shown to be active against most isolates of the following microorganisms both in vitro and in clinical infections, as described in the INDICATIONS AND USAGE section.
Aerobic and facultative Gram-positive microorganisms:
Enterococcus faecalis (vancomycin-susceptible isolates only)
Staphylococcus aureus (including methicillin-resistant isolates)
Streptococcus agalactiae
Streptococcus dysgalactiae subsp. equisimilis
Streptococcus pyogenes
The followingin vitro data are available, but their clinical significance is unknown. Greater than 90% of the following microorganisms demonstrate an in vitro MIC less than or equal to the susceptible breakpoint for daptomycin versus the bacterial genus. The efficacy of daptomycin in treating clinical infections due to these microorganisms has not been established in adequate and well-controlled clinical trials.
Aerobic and facultative Gram-positive microorganisms:
Corynebacterium jeikeium
Enterococcus faecalis (vancomycin-resistant isolates)
Enterococcus faecium (including vancomycin-resistant isolates)
Staphylococcus epidermidis (including methicillin-resistant isolates)
Staphylococcus haemolyticus
Susceptibility Testing Methods
Susceptibility testing by dilution methods requires the use of daptomycin susceptibility powder. The testing of daptomycin also requires the presence of physiological levels of free calcium ions (50 mg/L of calcium, using calcium chloride) in Mueller-Hinton broth medium.
Dilution Technique
Quantitative methods are used to determine antimicrobial MICs. These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure1, 2 based on a broth dilution method or equivalent using standardized inoculum and concentrations of daptomycin. The use of the agar dilution method is not recommended with daptomycin2. The MICs should be interpreted according to the criteria in Table 3.
Diffusion Technique
Quantitative methods that require measurement of zone diameters have not been shown to provide reproducible estimates of the susceptibility of bacteria to daptomycin. The use of the disk diffusion method is not recommended with daptomycin2, 3.
| Broth Dilution MIC | |||
| Pathogen | (µg/mL)a | ||
| S | I | R | |
| a. The MIC interpretive criteria for S. aureus and E. faecalis are applicable only to tests performed by broth dilution using Mueller-Hinton broth adjusted to a calcium content of 50 mg/L; the MIC interpretive criteria for Streptococcus spp. other than S. pneumoniae are applicable only to tests performed by broth dilution using Mueller-Hinton broth adjusted to a calcium content of 50 mg/L, supplemented with 2 to 5% lysed horse blood, inoculated with a direct colony suspension and incubated in ambient air at 35ºC for 20 to 24 hours. | |||
| b. The current absence of data on daptomycin-resistant isolates precludes defining any categories other than “Susceptible.” Isolates yielding test results suggestive of a “Non-Susceptible” category should be retested, and if the result is confirmed, the isolate should be submitted to a reference laboratory for further testing. | |||
| Staphylococcus aureus | ≤1 | (b) | (b) |
| (methicillin-susceptible and methicillin-resistant) | |||
| Streptococcus pyogenes, Streptococcus agalactiae, | ≤1 | (b) | (b) |
| and Streptococcus dysgalactiae subsp. equisimilis | |||
| Enterococcus faecalis | ≤4 | (b) | (b) |
| (vancomycin-susceptible only) | |||
A report of “Susceptible” indicates that the pathogen is likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable.
Quality Control
Standardized susceptibility test procedures require the use of quality control microorganisms to control the technical aspects of the procedures. Standard daptomycin powder should provide the range of values noted in Table 4. Quality control microorganisms are specific strains of organisms with intrinsic biological properties relating to resistance mechanisms and their genetic expression within bacteria; the specific strains used for microbiological quality control are not clinically significant.
| Quality Control Strain | Broth Dilution MIC Range |
| (μg/mL)a | |
| a. The quality control ranges for S. aureus and E. faecalis are applicable only to tests performed by broth dilution using Mueller-Hinton broth adjusted to a calcium content of 50 mg/L; the quality control ranges for S. pneumoniae are applicable only to tests performed by broth dilution using Mueller-Hinton broth adjusted to a calcium content of 50 mg/L, supplemented with 2 to 5% lysed horse blood, inoculated with a direct colony suspension and incubated in ambient air at 35ºC for 20 to 24 hours. | |
| b. This organism may be used for validation of susceptibility test results when testing Streptococcus spp. other than S. pneumoniae. | |
| Enterococcus faecalis ATCC 29212 | 1−4 |
| Staphylococcus aureus ATCC 29213 | 0.25−1 |
| Streptococcus pneumoniae ATCC 49619b | 0.06−0.5 |
INDICATIONS AND USAGE
CUBICIN (daptomycin for injection) is indicated for the following infections (see also DOSAGE AND ADMINISTRATION and CLINICAL STUDIES):
Complicated skin and skin structure infections (cSSSI) caused by susceptible isolates of the following Gram-positive microorganisms: Staphylococcus aureus (including methicillin-resistant isolates), Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus dysgalactiae subsp. equisimilis, and Enterococcus faecalis (vancomycin-susceptible isolates only). Combination therapy may be clinically indicated if the documented or presumed pathogens include Gram-negative or anaerobic organisms.
Staphylococcus aureus bloodstream infections (bacteremia), including those with right-sided infective endocarditis, caused by methicillin-susceptible and methicillin-resistant isolates. Combination therapy may be clinically indicated if the documented or presumed pathogens include Gram-negative or anaerobic organisms.
The efficacy of CUBICIN in patients with left-sided infective endocarditis due to S. aureus has not been demonstrated. The clinical trial of CUBICIN in patients with S. aureus bloodstream infections included limited data from patients with left-sided infective endocarditis; outcomes in these patients were poor (see CLINICAL STUDIES). CUBICIN has not been studied in patients with prosthetic valve endocarditis or meningitis.
Patients with persisting or relapsing S. aureus infection or poor clinical response should have repeat blood cultures. If a culture is positive for S. aureus, MIC susceptibility testing of the isolate should be performed using a standardized procedure, as well as diagnostic evaluation to rule out sequestered foci of infection (see PRECAUTIONS).
CUBICIN is not indicated for the treatment of pneumonia.
Appropriate specimens for microbiological examination should be obtained in order to isolate and identify the causative pathogens and to determine their susceptibility to daptomycin. Empiric therapy may be initiated while awaiting test results. Antimicrobial therapy should be adjusted as needed based upon test results.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of CUBICIN and other antibacterial drugs, CUBICIN should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
CONTRAINDICATIONS
CUBICIN is contraindicated in patients with known hypersensitivity to daptomycin.
WARNINGS
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including CUBICIN, and may range in severity from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of any antibacterial agent.
Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicated that a toxin produced by Clostridium difficile is a primary cause of “antibiotic-associated colitis.”
If a diagnosis of pseudomembranous colitis has been established, appropriate therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate-to-severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial agent clinically effective against C. difficile.
PRECAUTIONS
General
The use of antibiotics may promote the selection of non-susceptible organisms. Should superinfection occur during therapy, appropriate measures should be taken.
Prescribing CUBICIN in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Persisting or Relapsing S. aureus Infection
Patients with persisting or relapsing S. aureus infection or poor clinical response should have repeat blood cultures. If a culture is positive for S. aureus, MIC susceptibility testing of the isolate should be performed using a standardized procedure, as well as diagnostic evaluation to rule out sequestered foci of infection. Appropriate surgical intervention (e.g., debridement, removal of prosthetic devices, valve replacement surgery) and/or consideration of a change in antibiotic regimen may be required.
Failure of treatment due to persisting or relapsing S. aureus infections was assessed by the Adjudication Committee in 19/120 (15.8%) CUBICIN-treated patients (12 with MRSA and 7 with MSSA) and 11/115 (9.6%) comparator-treated patients (9 with MRSA treated with vancomycin and 2 with MSSA treated with anti-staphylococcal semi-synthetic penicillin). Among all failures, 6 CUBICIN-treated patients and 1 vancomycin-treated patient developed increasing MICs (reduced susceptibility) by central laboratory testing on or following therapy. Most patients who failed due to persisting or relapsing S. aureus infection had deep-seated infection and did not receive necessary surgical intervention (see CLINICAL STUDIES).
Skeletal Muscle
In a Phase 1 study examining doses up to 12 mg/kg q24h of CUBICIN for 14 days, no skeletal muscle effects or CPK elevations were observed.
In Phase 3 cSSSI trials of CUBICIN at a dose of 4 mg/kg, elevations in CPK were reported as clinical adverse events in 15/534 (2.8%) CUBICIN-treated patients, compared with 10/558 (1.8%) comparator-treated patients.
In the S. aureus bacteremia/endocarditis trial, at a dose of 6 mg/kg, elevations in CPK were reported as clinical adverse events in 8/120 (6.7%) CUBICIN-treated patients compared with 1/116 (<1%) comparator-treated patients. There were a total of 11 patients who experienced CPK elevations to above 500 U/L. Of these 11 patients, 4 had prior or concomitant treatment with an HMG-CoA reductase inhibitor.
Skeletal muscle effects associated with CUBICIN were observed in animals (see ANIMAL PHARMACOLOGY).
Patients receiving CUBICIN should be monitored for the development of muscle pain or weakness, particularly of the distal extremities. In patients who receive CUBICIN, CPK levels should be monitored weekly, and more frequently in patients who received recent prior or concomitant therapy with an HMG-CoA reductase inhibitor. In patients with renal insufficiency, both renal function and CPK should be monitored more frequently. Patients who develop unexplained elevations in CPK while receiving CUBICIN should be monitored more frequently. In the cSSSI studies, among patients with abnormal CPK (>500 U/L) at baseline, 2/19 (10.5%) treated with CUBICIN and 4/24 (16.7%) treated with comparator developed further increases in CPK while on therapy. In this same population, no patients developed myopathy. CUBICIN-treated patients with baseline CPK >500 U/L (N=19) did not experience an increased incidence of CPK elevations or myopathy relative to those treated with comparator (N=24). In the S. aureus bacteremia/endocarditis study, 3 (2.6%) CUBICIN-treated patients, including 1 with trauma associated with a heroin overdose and 1 with spinal cord compression, had an elevation in CPK >500 U/L with associated musculoskeletal symptoms. None of the patients in the comparator group had an elevation in CPK >500 U/L with associated musculoskeletal symptoms.
CUBICIN should be discontinued in patients with unexplained signs and symptoms of myopathy in conjunction with CPK elevation >1,000 U/L (~5X ULN), or in patients without reported symptoms who have marked elevations in CPK >2,000 U/L (≥10X ULN). In addition, consideration should be given to temporarily suspending agents associated with rhabdomyolysis, such as HMG-CoA reductase inhibitors, in patients receiving CUBICIN.
In a Phase 1 study examining doses up to 12 mg/kg q24h of CUBICIN for 14 days, no evidence of nerve conduction deficits or symptoms of periperal neuropathy was observed. In a small number of patients in Phase 1 and Phase 2 studies at doses up to 6 mg/kg, administration of CUBICIN was associated with decreases in nerve conduction velocity and with adverse events (e.g., paresthesias, Bell’s palsy) possibly reflective of peripheral or cranial neuropathy. Nerve conduction deficits were also detected in a similar number of comparator subjects in these studies. In Phase 3 cSSSI and community-acquired pneumonia (CAP) studies, 7/989 (0.7%) CUBICIN-treated patients and 7/1,018 (0.7%) comparator-treated patients experienced paresthesias. New or worsening peripheral neuropathy was not diagnosed in any of these patients. In the S. aureus bacteremia/endocarditis trial, a total of 11/120 (9.2%) CUBICIN-treated patients had treatment-emergent adverse events related to the peripheral nervous system. All of the events were classified as mild to moderate in severity; most were of short duration and resolved during continued treatment with CUBICIN or were likely due to an alternative etiology. In animals, effects of CUBICIN on peripheral nerve were observed (see ANIMAL PHARMACOLOGY). Therefore, physicians should be alert to the possibility of signs and symptoms of neuropathy in patients receiving CUBICIN.
Drug Interactions
Warfarin
Concomitant administration of CUBICIN (6 mg/kg q24h for 5 days) and warfarin (25 mg single oral dose) had no significant effect on the pharmacokinetics of either drug, and the INR was not significantly altered. As experience with the concomitant administration of CUBICIN and warfarin is limited, anticoagulant activity in patients receiving CUBICIN and warfarin should be monitored for the first several days after initiating therapy with CUBICIN (see CLINICAL PHARMACOLOGY, Drug-Drug Interactions).
HMG-CoA Reductase Inhibitors
Inhibitors of HMG-CoA reductase may cause myopathy, which is manifested as muscle pain or weakness associated with elevated levels of CPK. There were no reports of skeletal myopathy in a placebo-controlled Phase 1 trial in which 10 healthy subjects on stable simvastatin therapy were treated concurrently with CUBICIN (4 mg/kg q24h) for 14 days. In the Phase 3 S. aureus bacteremia/endocarditis trial, 5/22 CUBICIN-treated patients who received prior or concomitant therapy with an HMG-CoA reductase inhibitor developed CPK elevations >500 U/L. Experience with co-administration of HMG-CoA reductase inhibitors and CUBICIN in patients is limited; therefore, consideration should be given to temporarily suspending use of HMG-CoA reductase inhibitors in patients receiving CUBICIN (see ADVERSE REACTIONS, Post-Marketing Experience).
Drug-Laboratory Test Interactions
There are no reported drug-laboratory test interactions.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity studies in animals have not been conducted to evaluate the carcinogenic potential of daptomycin. However, neither mutagenic nor clastogenic potential was found in a battery of genotoxicity tests, including the Ames assay, a mammalian cell gene mutation assay, a test for chromosomal aberrations in Chinese hamster ovary cells, an in vivo micronucleus assay, an in vitro DNA repair assay, and an in vivo sister chromatid exchange assay in Chinese hamsters.
Daptomycin did not affect the fertility or reproductive performance of male and female rats when administered intravenously at doses up to 150 mg/kg/day, which is approximately 9 times the estimated human exposure level based upon AUCs.
Pregnancy
Teratogenic Effects: Pregnancy Category B
Reproductive and teratology studies performed in rats and rabbits at doses of up to 75 mg/kg, 2 and 4 times the 6 mg/kg human dose, respectively, on a body surface area basis, have revealed no evidence of harm to the fetus due to daptomycin. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
It is not known if daptomycin is excreted in human milk. Caution should be exercised when CUBICIN is administered to nursing women.
Pediatric Use
Safety and efficacy of CUBICIN in patients under the age of 18 have not been established.
Geriatric Use
Of the 534 patients treated with CUBICIN in Phase 3 controlled clinical trials of cSSSI, 27.0% were 65 years of age or older and 12.4% were 75 years of age or older. Of the 120 patients treated with CUBICIN in the Phase 3 controlled clinical trial of S. aureus bacteremia/endocarditis, 25.0% were 65 years of age or older and 15.8% were 75 years of age or older. In Phase 3 clinical studies of cSSSI and S. aureus bacteremia/endocarditis, lower clinical success rates were seen in patients ≥65 years of age compared with those <65 years of age. In addition, treatment-emergent adverse events were more common in patients ≥65 years old than in patients <65 years of age.
ANIMAL PHARMACOLOGY
In animals, daptomycin administration has been associated with effects on skeletal muscle with no changes in cardiac or smooth muscle. Skeletal muscle effects were characterized by degenerative/regenerative changes and variable elevations in CPK. No fibrosis or rhabdomyolysis was evident in repeat-dose studies up to the highest doses tested in rats (150 mg/kg/day) and dogs (100 mg/kg/day). The degree of skeletal myopathy showed no increase when treatment was extended from 1 month to up to 6 months. Severity was dose-dependent. All muscle effects, including microscopic changes, were fully reversible within 30 days following cessation of dosing.
In adult animals, effects on peripheral nerve (characterized by axonal degeneration and frequently accompanied by significant losses of patellar reflex, gag reflex, and pain perception) were observed at doses higher than those associated with skeletal myopathy. Deficits in the dogs’ patellar reflexes were seen within 2 weeks of the start of treatment at 40 mg/kg (9 times the human Cmax at the 6 mg/kg q24h dose), with some clinical improvement noted within 2 weeks of the cessation of dosing. However, at 75 mg/kg/day for 1 month, 7/8 dogs failed to regain full patellar reflex responses within the duration of a 3-month recovery period. In a separate study in dogs receiving doses of 75 and 100 mg/kg/day for 2 weeks, minimal residual histological changes were noted at 6 months after cessation of dosing. However, recovery of peripheral nerve function was evident.
Tissue distribution studies in rats have shown that daptomycin is retained in the kidney but appears to only minimally penetrate across the blood-brain barrier following single and multiple doses.
ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
Clinical studies sponsored by Cubist enrolled 1,667 patients treated with CUBICIN and 1,319 treated with comparator. Most adverse events reported in Cubist-sponsored Phase 1, 2, and 3 clinical studies were described as mild or moderate in intensity. In Phase 3 cSSSI trials, CUBICIN was discontinued in 15/534 (2.8%) patients due to an adverse event, while comparator was discontinued in 17/558 (3.0%) patients. In the S. aureus bacteremia/endocarditis trial, CUBICIN was discontinued in 20/120 (16.7%) patients due to an adverse event, while comparator was discontinued in 21/116 (18.1%) patients.
Gram-Negative Infections
In the S. aureus bacteremia/endocarditis trial, serious Gram-negative infections and nonserious Gram-negative bloodstream infections were reported in 10/120 (8.3%) CUBICIN-treated and 0/115 comparator-treated patients. Comparator patients received dual therapy that included initial gentamicin for 4 days. Events were reported during treatment and during early and late follow-up. Gram-negative infections included cholangitis, alcoholic pancreatitis, sternal osteomyelitis/mediastinitis, bowel infarction, recurrent Crohn’s disease, recurrent line sepsis, and recurrent urosepsis caused by a number of different Gram-negative organisms. One patient with sternal osteomyelitis following mitral valve repair developed S. aureus endocarditis with a 2 cm mitral vegetation and had a course complicated with bowel infarction, polymicrobial bacteremia, and death.
Other Adverse Reactions
The rates of most common adverse events, organized by body system, observed in cSSSI patients are displayed in Table 5.
| Adverse Event | CUBICIN 4 mg/kg | Comparator* |
| (N=534) | (N=558) | |
| * Comparators included vancomycin (1 g IV q12h) and anti-staphylococcal semi-synthetic penicillins (i.e., nafcillin, oxacillin, cloxacillin, flucloxacillin; 4 to 12 g/day IV in divided doses). | ||
| Gastrointestinal disorders | ||
| Constipation | 6.2% | 6.8% |
| Nausea | 5.8% | 9.5% |
| Diarrhea | 5.2% | 4.3% |
| Vomiting | 3.2% | 3.8% |
| Dyspepsia | 0.9% | 2.5% |
| General disorders | ||
| Injection site reactions | 5.8% | 7.7% |
| Fever | 1.9% | 2.5% |
| Nervous system disorders | ||
| Headache | 5.4% | 5.4% |
| Insomnia | 4.5% | 5.4% |
| Dizziness | 2.2% | 2.0% |
| Skin/subcutaneous disorders | ||
| Rash | 4.3% | 3.8% |
| Pruritus | 2.8% | 3.8% |
| Diagnostic investigations | ||
| Abnormal liver function tests | 3.0% | 1.6% |
| Elevated CPK | 2.8% | 1.8% |
| Infections | ||
| Fungal infections | 2.6% | 3.2% |
| Urinary tract infections | 2.4% | 0.5% |
| Vascular disorders | ||
| Hypotension | 2.4% | 1.4% |
| Hypertension | 1.1% | 2.0% |
| Renal/urinary disorders | ||
| Renal failure | 2.2% | 2.7% |
| Blood/lymphatic disorders | ||
| Anemia | 2.1% | 2.3% |
| Respiratory disorders | ||
| Dyspnea | 2.1% | 1.6% |
| Musculoskeletal disorders | ||
| Limb pain | 1.5% | 2.0% |
| Arthralgia | 0.9% | 2.2% |
Additional adverse events that occurred in 1 to 2% of patients in either CUBICIN (4 mg/kg) or comparator treatment groups in the cSSSI studies are as follows: edema, cellulitis, hypoglycemia, elevated alkaline phosphatase, cough, back pain, abdominal pain, hypokalemia, hyperglycemia, decreased appetite, anxiety, chest pain, sore throat, cardiac failure, confusion, and Candida infections. These events occurred at rates ranging from 0.2 to 1.7% in CUBICIN-treated patients and at rates of 0.4 to 1.8% in comparator-treated patients.
Additional drug-related adverse events (possibly or probably related) that occurred in <1% of patients receiving CUBICIN in the cSSSI trials are as follows:
Body as a Whole: fatigue, weakness, rigors, discomfort, jitteriness, flushing, hypersensitivity
Blood/Lymphatic System: leukocytosis, thrombocytopenia, thrombocytosis, eosinophilia, increased International Normalized Ratio (INR)
Cardiovascular System: supraventricular arrhythmia
Dermatologic System: eczema
Digestive System: abdominal distension, flatulence, stomatitis, jaundice, increased serum lactate dehydrogenase
Metabolic/Nutritional System: hypomagnesemia, increased serum bicarbonate, electrolyte disturbance
Musculoskeletal System: myalgia, muscle cramps, muscle weakness, osteomyelitis
Nervous System: vertigo, mental status change, paraesthesia
Special Senses: taste disturbance, eye irritation
The rates of most common adverse events, organized by System Organ Class (SOC), observed in S. aureus bacteremia/endocarditis (6 mg/kg CUBICIN) patients are displayed in Table 6.
| CUBICIN 6 mg/kg | Comparatora | |
| Adverse Event | (N=120) | (N=116) |
| n (%) | n (%) | |
| a. Comparator: vancomycin (1 g IV q12h) or anti-staphylococcal semi-synthetic penicillin (i.e., nafcillin, oxacillin, cloxacillin, flucloxacillin; 2 g IV q4h), each with initial low-dose gentamicin. | ||
| Infections and infestations | 65 (54.2%) | 56 (48.3%) |
| Urinary tract infection NOS | 8 (6.7%) | 11 (9.5%) |
| Osteomyelitis NOS | 7 (5.8%) | 7 (6.0%) |
| Sepsis NOS | 6 (5.0%) | 3 (2.6%) |
| Bacteraemia | 6 (5.0%) | 0 (0%) |
| Pneumonia NOS | 4 (3.3%) | 9 (7.8%) |
| Gastrointestinal disorders | 60 (50.0%) | 68 (58.6%) |
| Diarrhoea NOS | 14 (11.7%) | 21 (18.1%) |
| Vomiting NOS | 14 (11.7%) | 15 (12.9%) |
| Constipation | 13 (10.8%) | 14 (12.1%) |
| Nausea | 12 (10.0%) | 23 (19.8%) |
| Abdominal pain NOS | 7 (5.8%) | 4 (3.4%) |
| Dyspepsia | 5 (4.2%) | 8 (6.9%) |
| Loose stools | 5 (4.2%) | 6 (5.2%) |
| Gastrointestinal haemorrhage NOS | 2 (1.7%) | 6 (5.2%) |
| General disorders and administration site conditions | 53 (44.2%) | 69 (59.5%) |
| Oedema peripheral | 8 (6.7%) | 16 (13.8%) |
| Pyrexia | 8 (6.7%) | 10 (8.6%) |
| Chest pain | 8 (6.7%) | 7 (6.0%) |
| Oedema NOS | 8 (6.7%) | 5 (4.3%) |
| Asthenia | 6 (5.0%) | 6 (5.2%) |
| Injection site erythema | 3 (2.5%) | 7 (6.0%) |
| Respiratory, thoracic and mediastinal disorders | 38 (31.7%) | 43 (37.1%) |
| Pharyngolaryngeal pain | 10 (8.3%) | 2 (1.7%) |
| Pleural effusion | 7 (5.8%) | 8 (6.9%) |
| Cough | 4 (3.3%) | 7 (6.0%) |
| Dyspnoea | 4 (3.3%) | 6 (5.2%) |
| Skin and subcutaneous tissue disorders | 36 (30.0%) | 40 (34.5%) |
| Rash NOS | 8 (6.7%) | 10 (8.6%) |
| Pruritus | 7 (5.8%) | 6 (5.2%) |
| Erythema | 6 (5.0%) | 6 (5.2%) |
| Sweating increased | 6 (5.0%) | 0 (0%) |
| Musculoskeletal and connective tissue disorders | 35 (29.2%) | 42 (36.2%) |
| Pain in extremity | 11 (9.2%) | 11 (9.5%) |
| Back pain | 8 (6.7%) | 10 (8.6%) |
| Arthralgia | 4 (3.3%) | 13 (11.2%) |
| Psychiatric disorders | 35 (29.2%) | 28 (24.1%) |
| Insomnia | 11 (9.2%) | 8 (6.9%) |
| Anxiety | 6 (5.0%) | 6 (5.2%) |
| Nervous system disorders | 32 (26.7%) | 32 (27.6%) |
| Headache | 8 (6.7%) | 12 (10.3%) |
| Dizziness | 7 (5.8%) | 7 (6.0%) |
| Investigations | 30 (25.0%) | 33 (28.4%) |
| Blood creatine phosphokinase increased | 8 (6.7%) | 1 (<1%) |
| Blood and lymphatic system disorders | 29 (24.2%) | 24 (20.7%) |
| Anaemia NOS | 15 (12.5%) | 18 (15.5%) |
| Metabolism and nutrition disorders | 26 (21.7%) | 38 (32.8%) |
| Hypokalaemia | 11 (9.2%) | 15 (12.9%) |
| Hyperkalaemia | 6 (5.0%) | 10 (8.6%) |
| Vascular disorders | 21 (17.5%) | 20 (17.2%) |
| Hypertension NOS | 7 (5.8%) | 3 (2.6%) |
| Hypotension NOS | 6 (5.0%) | 9 (7.8%) |
| Renal and urinary disorders | 18 (15.0%) | 26 (22.4%) |
| Renal failure NOS | 4 (3.3%) | 11 (9.5%) |
| Renal failure acute | 4 (3.3%) | 7 (6.0%) |
The following events, not included above, were reported as possibly or probably drug-related in the CUBICIN-treated group:
Blood and Lymphatic System Disorders: eosinophilia (1.7%), lymphadenopathy (<1%), thrombocythaemia (<1%), thrombocytopenia (<1%)
Cardiac Disorders: atrial fibrillation (<1%), atrial flutter (<1%), cardiac arrest (<1%)
Ear and Labyrinth Disorders: tinnitus (<1%)
Eye Disorders: vision blurred (<1%)
Gastrointestinal Disorders: dry mouth (<1%), epigastric discomfort (<1%), gingival pain (<1%), hypoaesthesia oral (<1%)
Infections and Infestations: candidal infection NOS (1.7%), vaginal candidiasis (1.7%), fungaemia (<1%), oral candidiasis (<1%), urinary tract infection
fungal (<1%)
Investigations: blood phosphorous increased (2.5%), blood alkaline phosphatase increased (1.7%), INR ratio increased (1.7%), liver function
test abnormal (1.7%), alanine aminotransferase increased (<1%), aspartate aminotransferase increased (<1%), prothrombin time
prolonged (<1%)
Metabolism and Nutrition Disorders: appetite decreased NOS (<1%)
Musculoskeletal and Connective Tissue Disorders: myalgia (<1%)
Nervous System Disorders: dyskinesia (<1%), paraesthesia (<1%)
Psychiatric Disorders: hallucination NOS (<1%)
Renal and Urinary Disorders: proteinuria (<1%), renal impairment NOS (<1%)
Skin and Subcutaneous Tissue Disorders: heat rash (<1%), pruritus generalized (<1%), rash vesicular (<1%)
In Phase 3 studies of community-acquired pneumonia (CAP), the death rate and rates of serious cardiorespiratory adverse events were higher in CUBICIN-treated patients than in comparator-treated patients. These differences were due to lack of therapeutic effectiveness of CUBICIN in the treatment of CAP in patients experiencing these adverse events (see INDICATIONS AND USAGE).
Laboratory Changes
In Phase 3 comparator-controlled cSSSI and CAP studies, there was no clinically or statistically significant difference (p<0.05) in the incidence of CPK elevations between patients treated with CUBICIN and those treated with comparator. CPK elevations in both groups were generally related to medical conditions—for example, skin and skin structure infection, surgical procedures, or intramuscular injections—and were not associated with muscle symptoms.
In the Phase 3 cSSSI studies, 0.2% of patients treated with CUBICIN had symptoms of muscle pain or weakness associated with CPK elevations to greater than 4X ULN. The symptoms resolved within 3 days and CPK returned to normal within 7 to 10 days after discontinuing treatment (see PRECAUTIONS, Skeletal Muscle). Table 7 summarizes the CPK shifts from Baseline through End of Therapy in the cSSSI trials.
| All Patients | Patients with Normal CPK at Baseline | |||||||
| CUBICIN | Comparator | CUBICIN | Comparator | |||||
| Change | (N=430) | (N=459) | (N=374) | (N=392) | ||||
| % | N | % | N | % | N | % | N | |
| * ULN (Upper Limit of Normal) is defined as 200 U/L. | ||||||||
| Note: Elevations in CPK observed in patients treated with CUBICIN or comparator were not clinically or statistically significantly different. | ||||||||
| No Increase | 90.7% | 390 | 91.1% | 418 | 91.2% | 341 | 91.1% | 357 |
| Maximum Value >1X ULN* | 9.3% | 40 | 8.9% | 41 | 8.8% | 33 | 8.9% | 35 |
| >2X ULN | 4.9% | 21 | 4.8% | 22 | 3.7% | 14 | 3.1% | 12 |
| >4X ULN | 1.4% | 6 | 1.5% | 7 | 1.1% | 4 | 1.0% | 4 |
| >5X ULN | 1.4% | 6 | 0.4% | 2 | 1.1% | 4 | 0.0% | 0 |
| >10X ULN | 0.5% | 2 | 0.2% | 1 | 0.2% | 1 | 0.0% | 0 |
In the S. aureus bacteremia/endocarditis study, a total of 11 CUBICIN-treated patients (9.2%) had treatment-emergent elevations in CPK to >500 U/L, including 4 patients with elevations >10X ULN. Three of these 11 patients had CPK levels return to the normal range during continued CUBICIN treatment, 6 had values return to the normal range during follow-up, 1 had values returning toward baseline at the last assessment, and 1 did not have follow-up values reported. Three patients discontinued CUBICIN due to CPK elevation.
There was more renal dysfunction in comparator-treated patients than in CUBICIN-treated patients. The incidence of decreased renal function, defined as the proportion of patients with a creatinine clearance level <50 mL/min if baseline clearance was ≥50 mL/min or with a decrease of≥10 mL/min if baseline clearance was <50 mL/min, is shown in Table 8.
| CUBICIN 6 mg/kg | Comparatora | |
| Study Interval | (N=120) | (N=116) |
| n/N (%) | n/N (%) | |
| a. Comparator: vancomycin (1 g IV q12h) or anti-staphylococcal semi-synthetic penicillin (i.e., nafcillin, oxacillin, cloxacillin, flucloxacillin; 2 g IV q4h), each with initial low-dose gentamicin. | ||
| Days 2 to 4 | 2/96 (2.1%) | 6/90 (6.7%) |
| Days 2 to 7 | 6/115 (5.2%) | 16/113 (14.2%) |
| Day 2 to End of Therapy | 13/118 (11.0%) | 30/114 (26.3%) |
Post-Marketing Experience
The following adverse reactions have been reported with CUBICIN in worldwide post-marketing experience. Because these events are reported voluntarily from a population of unknown size, estimates of frequency cannot be made and causal relationship cannot be precisely established.
Immune System Disorders: anaphylaxis; hypersensitivity reactions, including pruritus, hives, shortness of breath, difficulty swallowing, and truncal
erythema.
Musculoskeletal System: rhabdomyolysis; some reports involved patients treated concurrently with CUBICIN and HMG-CoA reductase inhibitors.
OVERDOSAGE
In the event of overdosage, supportive care is advised with maintenance of glomerular filtration. Daptomycin is slowly cleared from the body by hemodialysis (approximately 15% recovered over 4 hours) or peritoneal dialysis (approximately 11% recovered over 48 hours). The use of high-flux dialysis membranes during 4 hours of hemodialysis may increase the percentage of dose removed compared with low-flux membranes.
DOSAGE AND ADMINISTRATION
Complicated Skin and Skin Structure Infections
CUBICIN 4 mg/kg should be administered over a 30-minute period by IV infusion in 0.9% sodium chloride injection once every 24 hours for 7 to 14 days. In Phase 1 and 2 clinical studies, CPK elevations appeared to be more frequent when CUBICIN was dosed more frequently than once daily. Therefore, CUBICIN should not be dosed more frequently than once a day.
Staphylococcus aureus Bloodstream Infections (Bacteremia), Including Those with Right-Sided Endocarditis, Caused by Methicillin-Susceptible and Methicillin-Resistant Strains
CUBICIN 6 mg/kg should be administered over a 30-minute period by IV infusion in 0.9% sodium chloride injection once every 24 hours for a minimum of 2 to 6 weeks. Duration of treatment should be based on the treating physician’s working diagnosis. There are limited safety data for the use of CUBICIN for more than 28 days of therapy. In the Phase 3 study, there were a total of 14 patients who were treated with CUBICIN for more than 28 days, 8 of whom were treated for 6 weeks or longer.
In Phase 1 and 2 clinical studies, CPK elevations appeared to be more frequent when CUBICIN was dosed more frequently than once daily. Therefore, CUBICIN should not be dosed more frequently than once a day.
Patients with Renal Impairment
Because daptomycin is eliminated primarily by the kidney, a dosage modification is recommended for patients with creatinine clearance <30 mL/min, including patients receiving hemodialysis or CAPD, as listed in Table 9. The recommended dosing regimen is 4 mg/kg (cSSSI) or 6 mg/kg (S. aureus bloodstream infections) once every 24 hours for patients with CLCR≥30 mL/min and 4 mg/kg (cSSSI) or 6 mg/kg (S. aureus bloodstream infections) once every 48 hours for patients with CLCR<30 mL/min, including those on hemodialysis or CAPD. In patients with renal insufficiency, both renal function and CPK should be monitored more frequently. When possible, CUBICIN should be administered following hemodialysis on hemodialysis days (see CLINICAL PHARMACOLOGY).
| Creatinine Clearance | Dosage Regimen | Dosage Regimen |
| (CLCR) | (cSSSI) | (S. aureus Bloodstream Infections) |
| ≥30 mL/min | 4 mg/kg once every 24 hours | 6 mg/kg once every 24 hours |
| <30 mL/min, including | 4 mg/kg once every 48 hours | 6 mg/kg once every 48 hours |
| hemodialysis or CAPD |
Preparation of CUBICIN for Administration
CUBICIN is supplied in single-use vials containing 500 mg daptomycin as a sterile, lyophilized powder. The contents of a CUBICIN 500 mg vial should be reconstituted with 10 mL of 0.9% sodium chloride injection. Reconstituted CUBICIN should be further diluted with 0.9% sodium chloride injection to be administered by IV infusion over a period of 30 minutes.
Since no preservative or bacteriostatic agent is present in this product, aseptic technique must be used in preparation of final IV solution. Stability studies have shown that the reconstituted solution is stable in the vial for 12 hours at room temperature or up to 48 hours if stored under refrigeration at 2 to 8ºC (36 to 46ºF). The diluted solution is stable in the infusion bag for 12 hours at room temperature or 48 hours if stored under refrigeration. The combined time (vial and infusion bag) at room temperature should not exceed 12 hours; the combined time (vial and infusion bag) under refrigeration should not exceed 48 hours.
CUBICIN vials are for single-use only.
Parenteral drug products should be inspected visually for particulate matter prior to administration.
Because only limited data are available on the compatibility of CUBICIN with other IV substances, additives or other medications should not be added to CUBICIN single-use vials or infused simultaneously through the same IV line. If the same IV line is used for sequential infusion of several different drugs, the line should be flushed with a compatible infusion solution before and after infusion with CUBICIN.
Compatible Intravenous Solutions
CUBICIN is compatible with 0.9% sodium chloride injection and lactated Ringer’s injection. CUBICIN is not compatible with dextrose-containing diluents.
HOW SUPPLIED
CUBICIN (daptomycin for injection) – Pale yellow to light brown lyophilized cake
Single-use 10 mL capacity vial, 500 mg/vial: Package of 1 (NDC 67919-011-01)
STORAGE
Store original packages at refrigerated temperatures, 2 to 8ºC (36 to 46ºF); avoid excessive heat.
CLINICAL STUDIES
Complicated Skin and Skin Structure Infections
Adult patients with clinically documented cSSSI (Table 10) were enrolled in two randomized, multinational, multicenter, investigator-blinded studies comparing CUBICIN (4 mg/kg IV q24h) with either vancomycin (1 g IV q12h) or an anti-staphylococcal semi-synthetic penicillin (i.e., nafcillin, oxacillin, cloxacillin, or flucloxacillin; 4 to 12 g IV per day). Patients known to have bacteremia at baseline were excluded. Patients with creatinine clearance (CLCR) between 30 and 70 mL/min were to receive a lower dose of CUBICIN as specified in the protocol; however, the majority of patients in this subpopulation did not have the dose of CUBICIN adjusted. Patients could switch to oral therapy after a minimum of 4 days of IV treatment if clinical improvement was demonstrated.
One study was conducted primarily in the United States and South Africa (study 9801), and the second (study 9901) was conducted at non-US sites only. Both studies were similar in design but differed in patient characteristics, including history of diabetes and peripheral vascular disease. There were a total of 534 patients treated with CUBICIN and 558 treated with comparator in the two studies. The majority (89.7%) of patients received IV medication exclusively.
The efficacy endpoints in both studies were the clinical success rates in the intent-to-treat (ITT) population and in the clinically evaluable (CE) population. In study 9801, clinical success rates in the ITT population were 62.5% (165/264) in patients treated with CUBICIN and 60.9% (162/266) in patients treated with comparator drugs. Clinical success rates in the CE population were 76.0% (158/208) in patients treated with CUBICIN and 76.7% (158/206) in patients treated with comparator drugs. In study 9901, clinical success rates in the ITT population were 80.4% (217/270) in patients treated with CUBICIN and 80.5% (235/292) in patients treated with comparator drugs. Clinical success rates in the CE population were 89.9% (214/238) in patients treated with CUBICIN and 90.4% (226/250) in patients treated with comparator drugs.
The success rates by pathogen for microbiologically evaluable patients are presented in Table 11.
| Study 9801 | Study 9901 | Pooled | |
| Primary Diagnosis | CUBICIN/Comparatora | CUBICIN/Comparatora | CUBICIN/Comparatora |
| N=264/N=266 | N=270/N=292 | N=534/N=558 | |
| a. Vancomycin or anti-staphylococcal semi-synthetic penicillins. | |||
| b. The majority of cases were subsequently categorized as complicated cellulitis, major abscesses, or traumatic wound infections. | |||
| Wound Infection | 99 (37.5%)/116 (43.6%) | 102 (37.8%)/108 (37.0%) | 201 (37.6%)/224 (40.1%) |
| Major Abscess | 55 (20.8%)/43 (16.2%) | 59 (21.9%)/65 (22.3%) | 114 (21.3%)/108 (19.4%) |
| Ulcer Infection | 71 (26.9%)/75 (28.2%) | 53 (19.6%)/68 (23.3%) | 124 (23.2%)/143 (25.6%) |
| Other Infectionb | 39 (14.8%)/32 (12.0%) | 56 (20.7%)/51 (17.5%) | 95 (17.8%)/83 (14.9%) |
| Success Rate | ||
| Pathogen | CUBICIN | Comparatora |
| n/N (%) | n/N (%) | |
| a. Vancomycin or anti-staphylococcal semi-synthetic penicillins. | ||
| b. As determined by the central laboratory. | ||
| Methicillin-susceptible Staphylococcus aureus (MSSA)b | 170/198 (85.9) | 180/207 (87.0) |
| Methicillin-resistant Staphylococcus aureus (MRSA)b | 21/28 (75.0) | 25/36 (69.4) |
| Streptococcus pyogenes | 79/84 (94.0) | 80/88 (90.9) |
| Streptococcus agalactiae | 23/27 (85.2) | 22/29 (75.9) |
| Streptococcus dysgalactiae subsp. equisimilis | 8/8 (100) | 9/11 (81.8) |
| Enterococcus faecalis (vancomycin-susceptible only) | 27/37 (73.0) | 40/53 (75.5) |
S. aureus Bacteremia/Endocarditis
The efficacy of CUBICIN in the treatment of patients with S. aureus bacteremia was demonstrated in a randomized, controlled, multinational, multicenter open-label study. In this study, adult patients with at least one positive blood culture for S. aureus obtained within 2 calendar days prior to the first dose of study drug and irrespective of source were enrolled and randomized to either CUBICIN (6 mg/kg IV q24h) or standard of care [anti-staphylococcal semi-synthetic penicillin 2 g IV q4h (nafcillin, oxacillin, cloxacillin, or flucloxacillin) or vancomycin 1 g IV q12h, both with initial gentamicin 1 mg/kg IV every 8 hours for first 4 days]. Of the patients in the comparator group, 93% received initial gentamicin for a median of 4 days compared with 1 patient (<1%) in the CUBICIN group. Patients with prosthetic heart valves, intravascular foreign material that was not planned for removal within 4 days after the first dose of study medication, severe neutropenia, known osteomyelitis, polymicrobial bloodstream infections, creatinine clearance <30 mL/min, and pneumonia were excluded.
Upon entry, patients were classified for likelihood of endocarditis using the modified Duke criteria (Possible, Definite, or Not Endocarditis). Echocardiography, including a transesophageal echocardiogram (TEE), was performed within 5 days following study enrollment. The choice of comparator agent was based on the oxacillin susceptibility of the S. aureus isolate. The duration of study treatment was based on the investigator’s clinical diagnosis. Final diagnoses and outcome assessments at Test of Cure (6 weeks after the last treatment dose) were made by a treatment-blinded Adjudication Committee, using protocol-specified clinical definitions and a composite primary efficacy endpoint (clinical and microbiological success) at the Test of Cure visit.
A total of 246 patients ≥18 years of age (124 CUBICIN, 122 comparator) with S. aureus bacteremia were randomized from 48 centers in the US and Europe. In the ITT population, 120 patients received CUBICIN and 115 received comparator (62 anti-staphylococcal semi-synthetic penicillin and 53 vancomycin). Thirty-five patients treated with anti-staphylococcal semi-synthetic penicillins received vancomycin initially for 1 to 3 days, pending final susceptibility results for the S. aureus isolates. The median age among the 235 patients in the ITT population was 53 years (range: 21 to 91 years); 30/120 (25%) in the CUBICIN group and 37/115 (32%) in the comparator group were ≥65 years of age. Of the 235 ITT patients, there were 141 (60%) males and 156 (66%) Caucasians across the two treatment groups. In addition, 176 (75%) of the ITT population had systemic inflammatory response syndrome (SIRS) and 85 (36%) had surgical procedures within 30 days of onset of the S. aureus bacteremia. Eighty-eight patients (38%) had bacteremia caused by MRSA. Entry diagnosis was based on the modified Duke criteria and included 37 (16%) Definite, 144 (61%) Possible, and 54 (23%) Not Endocarditis. Of the 37 patients with an entry diagnosis of Definite Endocarditis, all (100%) had a final diagnosis of infective endocarditis, and of the 144 patients with an entry diagnosis of Possible Endocarditis, 15 (10%) had a final diagnosis of infective endocarditis as assessed by the Adjudication Committee. Of the 54 patients with an entry diagnosis of Not Endocarditis, 1 (2%) had a final diagnosis of infective endocarditis as assessed by the Adjudication Committee.
There were 182 patients with bacteremia and 53 patients with infective endocarditis as assessed by the Adjudication Committee in the ITT population, including 35 with right-sided and 18 with left-sided endocarditis. The 182 patients with bacteremia included 121 with complicated and 61 with uncomplicated S. aureus bacteremia.
Complicated bacteremia was defined as S. aureus isolated from blood cultures obtained on at least 2 different calendar days, and/or metastatic foci of infection (deep tissue involvement), and classification of the patient as not having endocarditis according to the modified Duke criteria. Uncomplicated bacteremia was defined as S. aureus isolated from blood culture(s) obtained on a single calendar day, no metastatic foci of infection, no infection of prosthetic material, and classification of the patient as not having endocarditis according to the modified Duke criteria. The definition of right-sided endocarditis (RIE) used in the clinical trial was Definite or Possible Endocarditis according to the modified Duke criteria and no echocardiographic evidence of predisposing pathology or active involvement of either the mitral or aortic valve. Complicated RIE included patients who were not intravenous drug users, had a positive blood culture for MRSA, serum creatinine ≥2.5 mg/dL, or evidence of extrapulmonary sites of infection. Patients who were intravenous drug users, had a positive blood culture for MSSA, serum creatinine <2.5 mg/dL, and were without evidence of extrapulmonary sites of infection were considered to have uncomplicated RIE.
The co-primary efficacy endpoints in the study were the Adjudication Committee success rates at the Test of Cure visit (6 weeks after the last treatment dose) in the ITT and Per Protocol (PP) populations. The overall Adjudication Committee success rates in the ITT population were 44.2% (53/120) in patients treated with CUBICIN and 41.7% (48/115) in patients treated with comparator (difference = 2.4% [95% CI −10.2, 15.1]). The success rates in the PP population were 54.4% (43/79) in patients treated with CUBICIN and 53.3% (32/60) in patients treated with comparator (difference = 1.1% [95% CI −15.6, 17.8]).
Adjudication Committee success rates are shown in Table 12.
Eighteen (18/120) patients in the CUBICIN arm and 19/116 patients in the comparator arm died during the study. These include 3/28 CUBICIN-treated and 8/26 comparator-treated patients with endocarditis, as well as 15/92 CUBICIN-treated and 11/90 comparator-treated patients with bacteremia. Among patients with persisting or relapsing S. aureus infections, 8/19 CUBICIN-treated and 7/11 comparator-treated patients died.
Overall, there was no difference in time to clearance of S. aureus bacteremia between CUBICIN and comparator. The median time to clearance in patients with MSSA was 4 days and in patients with MRSA was 8 days.
Failure of treatment due to persisting or relapsing S. aureus infections was assessed by the Adjudication Committee in 19/120 (15.8%) CUBICIN-treated patients (12 with MRSA and 7 with MSSA) and 11/115 (9.6%) comparator-treated patients (9 with MRSA treated with vancomycin and 2 with MSSA treated with anti-staphylococcal semi-synthetic penicillin). Among all failures, 6 CUBICIN-treated patients and 1 vancomycin-treated patient developed increasing MICs (reduced susceptibility) by central laboratory testing on or following therapy. Most patients who failed due to persisting or relapsing S. aureus infection had deep-seated infection and did not receive necessary surgical intervention (see PRECAUTIONS).
| Difference: | |||
| Population | CUBICIN 6 mg/kg | Comparatora | CUBICIN − Comparator |
| n/N (%) | n/N (%) | (Confidence Interval) | |
| a. Comparator: vancomycin (1 g IV q12h) or anti-staphylococcal semi-synthetic penicillin (i.e., nafcillin, oxacillin, cloxacillin, flucloxacillin; 2 g IV q4h), each with initial low-dose gentamicin | |||
| b. According to the modified Duke criteria4 | |||
| c. 95% Confidence Interval | |||
| d. 97.5% Confidence Interval (adjusted for multiplicity) | |||
| e. 99% Confidence Interval (adjusted for multiplicity) | |||
| Overall | 53/120 (44.2%) | 48/115 (41.7%) | 2.4% (−10.2, 15.1)c |
| Baseline Pathogen | |||
| MSSA | 33/74 (44.6%) | 34/70 (48.6%) | −4.0% (−22.6, 14.6)d |
| MRSA | 20/45 (44.4%) | 14/44 (31.8%) | 12.6% (−10.2, 35.5)d |
| Entry Diagnosisb | |||
| Definite or Possible Infective | 41/90 (45.6%) | 37/91 (40.7%) | 4.9% (−11.6, 21.4)d |
| Endocarditis | |||
| Not Infective Endocarditis | 12/30 (40.0%) | 11/24 (45.8%) | −5.8% (−36.2, 24.5)d |
| Final Diagnosis | |||
| Uncomplicated Bacteremia | 18/32 (56.3%) | 16/29 (55.2%) | 1.1% (−31.7, 33.9)e |
| Complicated Bacteremia | 26/60 (43.3%) | 23/61 (37.7%) | 5.6% (−17.3, 28.6)e |
| Right-Sided Infective | 8/19 (42.1%) | 7/16 (43.8%) | −1.6% (−44.9, 41.6)e |
| Endocarditis | |||
| Uncomplicated Right-Sided | 3/6 (50.0%) | 1/4 (25.0%) | 25.0% (−51.6, 100.0)e |
| Infective Endocarditis | |||
| Complicated Right-Sided | 5/13 (38.5%) | 6/12 (50.0%) | −11.5% (−62.4, 39.4)e |
| Infective Endocarditis | |||
| Left-Sided Infective | 1/9 (11.1%) | 2/9 (22.2%) | −11.1% (−55.9, 33.6)e |
| Endocarditis | |||
REFERENCES
-
Clinical and Laboratory Standards Institute (CLSI). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—seventh edition. CLSI Document M7-A7; Wayne, PA. 2006 January.
-
Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; sixteenth informational supplement. CLSI Document M100-S16; Wayne, PA. 2006 January.
-
Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial disk susceptibility tests; approved standard—ninth edition. CLSI Document M2-A9; Wayne, PA. 2006 January.
-
Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, Bashore T, Corey GR. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633–638.
Rx only
US Patent Nos. 4,874,843; 4,885,243; 6,468,967; 6,696,412; 6,852,689; RE39,071
CUBICIN is a registered trademark of Cubist Pharmaceuticals, Inc. All other trademarks are property of their respective owners.
Manufactured for:
Cubist Pharmaceuticals, Inc.
Lexington, MA 02421 USA
Distributed by:
Integrated Commercialization Solutions (ICS)
Brooks, KY 40109 USA
For all medical inquiries call: (866) 793-2786
May 2006
| Cubicin (Daptomycin) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Revised: 07/2006Integrated Commercialization Solutions