EXALGO
-
hydromorphone hydrochloride tablet, extended release
MALLINCKRODT INC (BRAND PHARMACEUTICALS)
----------
|
||||||||||||||||||||||
FULL PRESCRIBING INFORMATION
WARNING: POTENTIAL FOR ABUSE, IMPORTANCE OF PROPER PATIENT
SELECTION AND LIMITATIONS OF USE
Potential for Abuse
EXALGO contains hydromorphone, an opioid agonist and a Schedule II controlled substance with an abuse liability similar to other opioid analgesics. EXALGO can be abused in a manner similar to other opioid agonists, legal or illicit. These risks should be considered when administering, prescribing, or dispensing EXALGO in situations where the healthcare professional is concerned about increased risk of misuse, abuse, or diversion. Schedule II opioid substances which include hydromorphone, morphine, oxycodone, fentanyl, oxymorphone and methadone have the highest potential for abuse and risk of fatal overdose due to respiratory depression [see Drug Abuse and Dependence (9)].
Proper Patient Selection
EXALGO is an extended-release formulation of hydromorphone hydrochloride indicated for the management of moderate to severe pain in opioid tolerant patients when a continuous around-the-clock opioid analgesic is needed for an extended period of time. Patients considered opioid tolerant are those who are taking at least 60 mg oral morphine per day, 25 mcg transdermal fentanyl/hour, 30 mg oral oxycodone/day, 8 mg oral hydromorphone/day, 25 mg oral oxymorphone/day or an equianalgesic dose of another opioid, for a week or longer [see Indications and Usage (1) and Dosage and Administration (2)].
EXALGO is for use in opioid tolerant patients only [see Indications and Usage (1) and Dosage and Administration (2)].
Fatal respiratory depression could occur in patients who are not opioid tolerant.
Accidental consumption of EXALGO, especially in children, can result in a fatal overdose of hydromorphone [see Warnings and Precautions (5.1)].
Limitations of Use
EXALGO is not indicated for the management of acute or postoperative pain [see Indications and Usage (1)].
EXALGO is not intended for use as an as-needed analgesic [see Indications and Usage (1)].
EXALGO tablets are to be swallowed whole and are not to be broken, chewed, dissolved, crushed or injected. Taking broken, chewed, dissolved or crushed EXALGO or its contents leads to rapid release and absorption of a potentially fatal dose of hydromorphone [see Warnings and Precautions (5)].
1 INDICATIONS AND USAGE
EXALGO is an extended-release oral formulation of hydromorphone hydrochloride indicated for the management of moderate to severe pain in opioid tolerant patients requiring continuous, around-the-clock opioid analgesia for an extended period of time.
Patients considered opioid tolerant are those who are taking at least 60 mg oral morphine per day, 25 mcg transdermal fentanyl/hour, 30 mg oral oxycodone/day, 8 mg oral hydromorphone/day, 25 mg oral oxymorphone/day or an equianalgesic dose of another opioid, for a week or longer.
EXALGO is NOT intended for use as an as-needed analgesic.
EXALGO is not indicated for the management of acute or postoperative pain.
2 DOSAGE AND ADMINISTRATION
Selection of patients for treatment with EXALGO is governed by the same principles that apply to the use of similar opioid analgesics. Physicians should individualize treatment in every case, using non-opioid analgesics, opioids on an as-needed basis and/or combination products, and chronic opioid therapy in a progressive plan of pain management such as the guidelines outlined by the World Health Organization or the Federation of State Medical Boards Model Guidelines.
EXALGO should be swallowed whole and should not be broken, crushed, dissolved, or chewed before swallowing.
2.1 Conversion to EXALGO in Opioid Tolerant Patients
The dose range of EXALGO studied in clinical trials is 8 mg to 64 mg. The tablets are to be administered every 24 hours with or without food. Discontinue all other extended-release opioids when beginning EXALGO therapy. As EXALGO is only for use in opioid tolerant patients, do not begin any patient on EXALGO as the first opioid.
Use caution to avoid medication errors when prescribing or dispensing EXALGO 8 mg tablets, as 8 mg tablets are also available as immediate-release hydromorphone tablets.
It is critical to initiate the dosing regimen individually for each patient. Overestimating the EXALGO dose when converting patients from another opioid medication can result in fatal overdose with the first dose [see Overdosage (10)].
In the selection of the initial dose of EXALGO, give attention to the following:
- the daily dose, potency, and specific characteristics of the opioid the patient has been taking previously;
- the reliability of the relative potency estimate used to calculate the equivalent hydromorphone dose needed;
- the patient's degree of opioid tolerance;
- the age, general condition, and medical status of the patient;
- concurrent non-opioid analgesics and other medications, such as those with Central Nervous System (CNS) activity [see Drug Interactions (7)];
- the type and severity of the patient's pain;
- the balance between pain control and adverse effects;
- risk factors for abuse, addiction, or diversion, including a prior history of abuse, addiction, or diversion.
The following dosing recommendations, therefore, can only be considered as suggested approaches to what is actually a series of clinical decisions over time in the management of the pain of each individual patient.
Conversion from Other Oral Hydromorphone Formulations to EXALGO
Patients receiving oral immediate-release hydromorphone may be converted to EXALGO by administering a starting dose equivalent to the patient’s total daily oral hydromorphone dose, taken once daily. The dose of EXALGO can be titrated every 3 to 4 days until adequate pain relief with tolerable side effects has been achieved [see Dosage and Administration (2.1)].
Conversion from Oral Opioids to EXALGO
For conversion from other opioids to EXALGO, physicians and other healthcare professionals are advised to refer to published relative potency information, keeping in mind that conversion ratios are only approximate. In general, start EXALGO therapy by administering 50% of the calculated total daily dose of EXALGO (see conversion ratio table below) every 24 hours. The initial dose of EXALGO can be titrated until adequate pain relief with tolerable side effects has been achieved. The following table provides approximate equivalent doses, which may be used as a guideline for conversion.
- The conversion ratios and approximate equivalent doses in this conversion table (Table 1) are only to be used for the conversion from current oral opioid therapy to EXALGO. No fixed conversion ratio is likely to be satisfactory in all patients, especially in patients receiving large opioid doses.
- For patients on a regimen of mixed opioids, calculate the approximate oral hydromorphone dose for each opioid and sum the totals.
- For patients on a regimen of fixed-ratio opioid/non-opioid analgesic medications, only the opioid component of these medications should be used in the conversion. The non-opioid component may be continued as a separate drug, or a different non-opioid analgesic may be selected.
- There is substantial patient variation in the relative potency of different opioid drugs and formulations.
- It is extremely important to monitor all patients closely when converting from methadone to other opioid agonists. The ratio between methadone and other opioid agonists may vary widely as a function of previous dose exposure. Methadone has a long half-life and tends to accumulate in the plasma.
- The recommended doses are only a starting point, and close observation and titration are indicated until a satisfactory dose is obtained on the new therapy.
| Previous Opioid | Approximate Equivalent Oral Dose | Oral Conversion Ratio† |
|---|---|---|
|
||
| Hydromorphone | 12 mg | 1 |
| Codeine | 200 mg | 0.06 |
| Hydrocodone | 30 mg | 0.4 |
| Methadone‡ | 20 mg | 0.6 |
| Morphine | 60 mg | 0.2 |
| Oxycodone | 30 mg | 0.4 |
| Oxymorphone | 20 mg | 0.6 |
Select opioid, sum the total daily dose, and then multiply the dose by the conversion ratio to calculate the approximate oral hydromorphone equivalent.
Conversion from Transdermal Fentanyl to EXALGO
Eighteen hours following the removal of the transdermal fentanyl patch, EXALGO treatment can be initiated. For each 25 mcg/hr fentanyl transdermal dose the equianalgesic dose of EXALGO is 12 mg every 24 hours. An appropriate starting dose of EXALGO is 50% of the calculated total daily dose every 24 hours.
- Once therapy is initiated, assess pain relief and other opioid adverse reactions frequently.
- Titrate patients to adequate analgesia with dose increases not more often than every 3 to 4 days, in order to attain steady-state plasma concentrations of hydromorphone at each dose.
- As a guideline, consider dosage increases of 25% to 50% of the current daily dose of EXALGO for each titration step.
- If more than two doses of rescue medication are needed within a 24 hour period for two consecutive days, the dose of EXALGO may need to be titrated upward.
- Administer EXALGO no more frequently than every 24 hours.
During periods of changing analgesic requirements, including initial titration, maintain frequent contact between physician, other members of the healthcare team, the patient and the caregiver/family.
2.2 Maintenance of Therapy
During chronic therapy with EXALGO, assess the continued need for around-the-clock opioid therapy periodically. Continue to assess patients for their clinical risks for opioid abuse, addiction, or diversion particularly with high-dose formulations. If patients need to titrate while on maintenance therapy, follow the same method outlined in Individualization of Dosage to re-establish pain control.
2.3 Discontinuation of EXALGO
When the patient no longer requires therapy with EXALGO, taper doses gradually, by 25 to 50% every 2 or 3 days down to a dose of 8 mg before discontinuation of therapy, to prevent signs and symptoms of withdrawal in the physically dependent patient.
2.4 Renal and Hepatic Impairment
Start patients with moderate and severe hepatic and moderate renal impairment on a reduced dose and closely monitor during dose titration. As EXALGO is only intended for once daily administration, consider use of an alternate analgesic that may permit more flexibility with the dosing interval in patients with severe renal impairment.
3 DOSAGE FORMS AND STRENGTHS
EXALGO is available in 8 mg, 12 mg or 16 mg dosage strengths. The 8 mg tablets are round, biconvex, red tablets imprinted with "EXH 8" on one side. The 12 mg tablets are round, biconvex, dark yellow tablets imprinted with "EXH 12" on one side. The 16 mg tablets are round, biconvex, yellow tablets imprinted with "EXH 16" on one side.
4 CONTRAINDICATIONS
4.1 Opioid Non-Tolerant Patients
EXALGO is contraindicated in opioid non-tolerant patients. Fatal respiratory depression could occur in patients who are not opioid tolerant.
4.2 Impaired Pulmonary Function
EXALGO is contraindicated in patients with significant respiratory depression, especially in the absence of resuscitative equipment or in unmonitored settings and in patients with acute or severe bronchial asthma or hypercarbia.
4.3 Paralytic Ileus
EXALGO is contraindicated in patients who have or are suspected of having a paralytic ileus.
4.4 Preexisting Gastrointestinal (GI) Surgery or Narrowing of GI Tract
EXALGO is contraindicated in patients who have had surgical procedures and/or underlying disease that would result in narrowing of the gastrointestinal tract, or have “blind loops” of the gastrointestinal tract or gastrointestinal obstruction.
4.5 Allergy or Hypersensitivity
EXALGO is contraindicated in patients with known hypersensitivity to any of its components including the active agent, hydromorphone hydrochloride or known allergy to sulfite-containing medications [see Warnings and Precautions (5.8)].
5 WARNINGS AND PRECAUTIONS
5.1 Information Essential for Safe Administration
EXALGO tablets are to be swallowed whole, and are not to be broken, chewed, crushed, dissolved or injected. Taking broken, chewed, crushed, dissolved EXALGO or its contents leads to the rapid release and absorption of a potentially fatal dose of hydromorphone [see Boxed Warning].
EXALGO is for use only in opioid tolerant patients. Ingestion of EXALGO may cause fatal respiratory depression when administered to patients who are not opioid tolerant [see Boxed Warning].
EXALGO tablets must be kept in a secure place out of the reach of children. Accidental consumption of EXALGO, especially in children, can result in a fatal overdose of hydromorphone.
5.2 Misuse and Abuse
EXALGO contains hydromorphone, an opioid agonist, and is a Schedule II controlled substance. Opioid agonists have the potential for being abused and are sought by drug abusers and people with addiction disorders and are subject to criminal diversion.
EXALGO can be abused in a manner similar to other opioid agonists, legal or illicit. This should be considered when prescribing or dispensing EXALGO in situations where the healthcare professional is concerned about an increased risk of misuse, abuse, or diversion.
Breaking, crushing, chewing, or dissolving the contents of an EXALGO tablet results in the uncontrolled delivery of the opioid and poses a significant risk of overdose and death [see Drug Abuse and Dependence (9)].
If attempts are made to extract the drug from the hard outer shell for purposes of parenteral abuse, the injection of tablet excipients may be toxic and may result in lethal complications.
Concerns about abuse, addiction, and diversion should not prevent the proper management of pain. However, all patients treated with opioids, including EXALGO, require careful monitoring for signs of abuse and addiction, since use of opioid analgesic products carries the risk of addiction even under appropriate medical use.
Healthcare professionals should contact their State Professional Licensing Board or State Controlled Substances Authority for information on how to prevent and detect abuse or diversion of this product.
5.3 Respiratory Depression
Respiratory depression is the chief hazard of EXALGO. Respiratory depression occurs more frequently in elderly or debilitated patients as well as those suffering from conditions accompanied by hypoxia or hypercapnia when even moderate therapeutic doses may dangerously decrease pulmonary ventilation, and when opioids are given in conjunction with other agents that depress respiration.
Use EXALGO with extreme caution in patients with conditions accompanied by hypoxia, hypercapnia, or decreased respiratory reserve such as asthma, chronic obstructive pulmonary disease or cor pulmonale, severe obesity, sleep apnea, myxedema, kyphoscoliosis or CNS depression. In these patients, even moderate therapeutic doses of hydromorphone may decrease respiratory drive while simultaneously increasing airway resistance to the point of apnea. In these patients, consider alternative non-opioid analgesics, and use EXALGO only under careful medical supervision at the lowest effective dose.
5.4 Interactions with Alcohol and Other CNS Depressants
The concurrent use of EXALGO with other central nervous system (CNS) depressants, including but not limited to other opioids, illicit drugs, sedatives, hypnotics, general anesthetics, phenothiazines, muscle relaxants, other tranquilizers, and alcohol, increases the risk of respiratory depression, hypotension, and profound sedation, potentially resulting in coma or death. Use with caution and in reduced dosages in patients taking CNS depressants. Avoid concurrent use of alcohol and EXALGO [see Clinical Pharmacology (12.3)].
5.5 Head Injury and Increased Intracranial Pressure
In the presence of head injury, intracranial lesions or a preexisting increase in intracranial pressure, the respiratory depressant effects of EXALGO and its potential to elevate cerebrospinal fluid pressure (resulting from vasodilation following CO2 retention) may be markedly exaggerated. Furthermore, EXALGO can produce effects on pupillary response and consciousness, which may obscure neurologic signs of further increases in intracranial pressure in patients with head injuries.
5.6 Hypotensive Effect
EXALGO may cause severe hypotension. There is added risk to individuals whose ability to maintain blood pressure has been compromised by a depleted blood volume, or after concurrent administration with drugs such as phenothiazines, general anesthetics, or other agents that compromise vasomotor tone.
Administer EXALGO with caution to patients in circulatory shock, since vasodilation produced by the drug may further reduce cardiac output and blood pressure.
5.7 Gastrointestinal Effects
Because the EXALGO tablet is nondeformable and does not appreciably change in shape in the GI tract, do not administer EXALGO to patients with preexisting severe gastrointestinal narrowing (pathologic or iatrogenic, for example: esophageal motility disorders small bowel inflammatory disease, “short gut” syndrome due to adhesions or decreased transit time, past history of peritonitis, cystic fibrosis, chronic intestinal pseudoobstruction, or Meckel’s diverticulum). There have been reports of obstructive symptoms in patients with known strictures or risk of strictures, such as previous GI surgery, in association with the ingestion of drugs in nondeformable extended-release formulations.
The administration of EXALGO may obscure the diagnosis or clinical course in patients with acute abdominal condition.
It is possible that EXALGO tablets may be visible on abdominal x-rays under certain circumstances, especially when digital enhancing techniques are utilized.
5.8 Sulfites
EXALGO contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
5.9 MAO Inhibitors
EXALGO is not recommended for use in patients who have received MAO inhibitors within 14 days, because severe and unpredictable potentiation by MAO inhibitors has been reported with opioid analgesics.
5.10 Special Risk Groups
EXALGO should be administered with caution in elderly (≥ 65 years) and debilitated patients and in patients who are known to be sensitive to central nervous system depressants, such as those with cardiovascular, pulmonary, renal, or hepatic disease [see Use in Specific Populations (8)].
EXALGO should also be used with caution in the following conditions: adrenocortical insufficiency (e.g., Addison's disease); delirium tremens; myxedema or hypothyroidism; prostatic hypertrophy or urethral stricture; and, toxic psychosis. EXALGO may aggravate convulsions in patients with convulsive disorders, and all opioids may induce or aggravate seizures in some clinical settings.
5.11 Use in Pancreatic/Biliary Tract Disease
EXALGO can cause an increase in biliary tract pressure as a result of spasm in the sphincter of Oddi. Caution should be exercised in the administration of EXALGO to patients with inflammatory or obstructive bowel disorders, acute pancreatitis secondary to biliary tract disease and in patients about to undergo biliary surgery.
5.12 Driving and Operating Machinery
EXALGO may impair the mental and/or physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. Caution patients accordingly. Also warn patients about the potential combined effects of EXALGO with other CNS depressants, including other opioids, phenothiazines, sedative/hypnotics, and alcohol [see Drug Interactions (7)].
5.13 Precipitation of Withdrawal
Mixed agonist/antagonist analgesics (i.e., pentazocine, nalbuphine, and butorphanol) should not be administered to patients who have received or are receiving a course of therapy with a pure opioid agonist analgesic, including EXALGO. In these patients, mixed agonists/antagonists analgesics may reduce the analgesic effect and/or may precipitate withdrawal symptoms. Do not abruptly discontinue EXALGO.
Clinical conditions or medicinal products that cause a sudden and significant shortening of gastrointestinal transit time may result in decreased hydromorphone absorption with EXALGO and may potentially lead to withdrawal symptoms in patients with a physical dependence on opioids.
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the labeling:
- Respiratory Depression [see Warnings and Precautions (5.3)]
- Head Injury and Increased Intracranial Pressure [see Warnings and Precautions (5.5)]
- Hypotensive Effect [see Warnings and Precautions (5.6)]
- Gastrointestinal Effects [see Warnings and Precautions (5.7)]
- Cardiac Arrest [see Overdosage (10)]
- Precipitation of Withdrawal [see Warnings and Precautions (5.13)]
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
EXALGO was administered to a total of 2,524 patients in 15 controlled and uncontrolled clinical studies. Of these, 423 patients were exposed to EXALGO for greater than 6 months and 141 exposed for greater than one year.
The overall incidence of adverse reactions in patients greater than 65 years of age was higher, with a greater than 5% difference in rates for constipation and nausea when compared with younger patients. The overall incidence of adverse reactions in female patients was higher, with a greater than 5% difference in rates for nausea, vomiting, constipation and somnolence when compared with male patients.
A 12-week double-blind, placebo-controlled, randomized withdrawal study was conducted in opioid tolerant patients with moderate to severe low back pain [see Clinical Studies (14)]. A total of 447 patients were enrolled into the open-label titration phase with 268 patients randomized into the double-blind treatment phase. The adverse reactions that were reported in at least 2% of the patients are contained in Table 2.
| Preferred Term | Open-Label Titration Phase | Double-Blind Treatment Phase | |
|---|---|---|---|
| EXALGO (N = 447) | EXALGO (N = 134) | Placebo (N = 134) |
|
| Constipation | 69 (15) | 10 (7) | 5 (4) |
| Nausea | 53 (12) | 12 (9) | 10 (7) |
| Somnolence | 39 (9) | 1 (1) | 0 (0) |
| Headache | 35 (8) | 7 (5) | 10 (7) |
| Vomiting | 29 (6) | 8 (6) | 6 (4) |
| Drug Withdrawal Syndrome | 22 (5) | 13 (10) | 16 (12) |
| Pruritus | 21 (5) | 1 (1) | 0 (0) |
| Dizziness | 17 (4) | 3 (2) | 2 (1) |
| Asthenia* | 16 (4) | 2 (1) | 6 (4) |
| Insomnia | 13 (3) | 7 (5) | 5 (4) |
| Diarrhea | 13 (3) | 5 (4) | 9 (7) |
| Back Pain | 13 (3) | 6 (4) | 8 (6) |
| Dry Mouth | 13 (3) | 2 (1) | 0 (0) |
| Edema Peripheral | 13 (3) | 3 (2) | 1 (1) |
| Hyperhidrosis | 13 (3) | 2 (1) | 2 (1) |
| Anorexia† | 10 (2) | 2 (1) | 0 (0) |
| Arthralgia | 9 (2) | 8 (6) | 3 (2) |
| Anxiety | 9 (2) | 0 (0) | 4 (3) |
| Abdominal pain‡ | 9 (2) | 4 (3) | 3 (2) |
| Muscle Spasms | 5 (1) | 3 (2) | 1 (1) |
| Weight Decreased | 3 (1) | 4 (3) | 3 (2) |
The adverse reactions that were reported in at least 2% of the total treated patients (N=2,474) in the 14 chronic clinical trials are contained in Table 3.
| Preferred Term | All Patients (N = 2,474) | |
|---|---|---|
|
||
| Constipation | 765 (31) | |
| Nausea | 684 (28) | |
| Vomiting | 337 (14) | |
| Somnolence | 367 (15) | |
| Headache | 308 (12) | |
| Asthenia* | 272 (11) | |
| Dizziness | 262 (11) | |
| Diarrhea | 201 (8) | |
| Pruritus | 193 (8) | |
| Insomnia | 161 (7) | |
| Hyperhidrosis | 143 (6) | |
| Edema Peripheral | 135 (5) | |
| Anorexia† | 139 (6) | |
| Dry Mouth | 121 (5) | |
| Abdominal Pain‡ | 115 (5) | |
| Anxiety | 95 (4) | |
| Back Pain | 95 (4) | |
| Dyspepsia§ | 88 (4) | |
| Depression | 81 (3) | |
| Dyspnea¶ | 76 (3) | |
| Muscle Spasms | 74 (3) | |
| Arthralgia | 72 (3) | |
| Rash | 64 (3) | |
| Pain in Extremity | 63 (3) | |
| Pain | 58 (2) | |
| Drug Withdrawal Syndrome | 55 (2) | |
| Pyrexia | 52 (2) | |
| Fall | 51 (2) | |
| Chest discomfort# | 51 (2) | |
The following Adverse Reactions occurred in patients with an overall frequency of <2% and are listed in descending order within each System Organ Class:
Cardiac disorders: palpitations, tachycardia, bradycardia, extrasystoles
Ear and labyrinth disorders: vertigo, tinnitus
Endocrine disorders: hypogonadism
Eye disorders: vision blurred, diplopia, dry eye, miosis
Gastrointestinal disorders: flatulence, dysphagia, hematochezia, abdominal distension, hemorrhoids, abnormal feces, intestinal obstruction, eructation, diverticulum, gastrointestinal motility disorder, large intestine perforation, anal fissure, bezoar, duodenitis, ileus, impaired gastric emptying, painful defecation
General disorders and administration site conditions: chills, malaise, feeling abnormal, feeling hot and cold, feeling jittery, hangover, difficulty in walking, feeling drunk, hypothermia
Infections and infestations: gastroenteritis, diverticulitis
Injury, poisoning and procedural complications: contusion, overdose
Investigations: weight decreased, hepatic enzyme increased, blood potassium decreased, blood amylase increased, blood testosterone decreased, oxygen saturation decreased
Metabolism and nutrition disorders: dehydration, fluid retention, increased appetite, hyperuricemia
Musculoskeletal and connective tissue disorders: myalgia
Nervous system disorders: tremor, sedation, hypoesthesia, paraesthesia, disturbance in attention, memory impairment, dysarthria, syncope, balance disorder, dysgeusia, depressed level of consciousness, coordination abnormal, hyperesthesia, myoclonus, dyskinesia, hyperreflexia, encephalopathy, cognitive disorder, convulsion, psychomotor hyperactivity
Psychiatric disorders: confusional state, nervousness, restlessness, abnormal dreams, mood altered, hallucination, panic attack, euphoric mood, paranoia, dysphoria, listless, crying, suicide ideation, libido decreased, aggression
Renal and urinary disorders: dysuria, urinary retention, urinary frequency, urinary hesitation, micturition disorder
Reproductive system and breast disorders: erectile dysfunction, sexual dysfunction
Respiratory, thoracic and mediastinal disorders: rhinorrhoea, respiratory distress, hypoxia, bronchospasm, sneezing, hyperventilation, respiratory depression
Skin and subcutaneous tissue disorders: erythema
Vascular disorders: flushing, hypertension, hypotension
7 DRUG INTERACTIONS
7.1 CNS Depressants
The concomitant use of EXALGO with central nervous system depressants such as hypnotics, sedatives, general anesthetics, antipsychotics and alcohol may cause additive depressant effects and respiratory depression. Additionally, hypotension and profound sedation or coma could occur. When this combination is indicated, the dose of one or both agents should be reduced. The concomitant use of alcohol should be avoided [see Clinical Pharmacology (12.3)].
7.2 Monoamine Oxidase (MAO) Inhibitors
MAO inhibitors may cause CNS excitation or depression, hypotension or hypertension if co-administered with opioids including EXALGO. EXALGO is not intended for patients taking MAO inhibitors or within 14 days of stopping such treatment.
7.3 Mixed Agonist/Antagonist Opioid Analgesics
The concomitant use of EXALGO with morphine agonist/antagonists (buprenorphone, nalbuphine, pentazocine) could lead to a reduction of the analgesic effect by competitive blocking of receptors, thus leading to risk of withdrawal symptoms. Therefore, this combination is not recommended.
7.4 Anticholinergics
Anticholinergics or other medications with anticholinergic activity when used concurrently with EXALGO may result in increased risk of urinary retention and/or severe constipation, which may lead to paralytic ileus.
7.5 Cytochrome P450 Enzymes
In vitro data suggest that hydromorphone in clinically relevant concentrations has minimal potential to inhibit the activity of human hepatic CYP450 enzymes including CYP1A2, 2C9, 2C19, 2D6, 3A4, and 4A11.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects
Pregnancy Category C: There are no adequate and well-controlled studies in pregnant women. Hydromorphone crosses the placenta. EXALGO should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus [see Use in Specific Populations (8.2)].
Hydromorphone was not teratogenic in pregnant rats given oral doses up to 6.25 mg/kg/day or in pregnant rabbits administered oral doses up to 25 mg/kg/day during the period of organogenesis (~1.2 times the human exposure following 32 mg/day).
Hydromorphone administration to pregnant Syrian hamsters and CF-1 mice during major organ development revealed teratogenicity likely the result of maternal toxicity associated with sedation and hypoxia. In Syrian hamsters given single subcutaneous doses from 14 to 258 mg/kg during organogenesis (gestation days 8 to 10), doses ≥ 19 mg/kg hydromorphone produced skull malformations (exencephaly and cranioschisis). Continuous infusion of hydromorphone (5 mg/kg, s.c.) via implanted osmotic mini pumps during organogenesis (gestation days 7 to 10) produced soft tissue malformations (cryptorchidism, cleft palate, malformed ventricals and retina), and skeletal variations (supraoccipital, checkerboard and split sternebrae, delayed ossification of the paws and ectopic ossification sites). The malformations and variations observed in the hamsters and mice were at doses approximately three-fold higher and <one-fold lower, respectively, than a 32 mg human daily oral dose on a body surface area basis.
Nonteratogenic Effects
In the pre- and post-natal effects study in rats, neonatal viability was reduced at 6.25 mg/kg/day (~1.2 times the human exposure following 32 mg/day).
Neonates born to mothers who have been taking opioids regularly prior to delivery will be physically dependent. The withdrawal signs include irritability and excessive crying, tremors, hyperactive reflexes, increased respiratory rate, increased stools, sneezing, yawning, vomiting, and fever. The intensity of the syndrome does not always correlate with the duration of maternal opioid use or dose. There is no consensus on the best method of managing withdrawal. Approaches to the treatment of the syndrome have included supportive care and, if indicated, drugs such as paregoric or phenobarbital.
8.2 Labor and Delivery
EXALGO is not recommended for use in women during and immediately prior to labor and delivery. Administration of EXALGO to the mother shortly before delivery may result in some degree of respiratory depression in the neonate. However, neonates whose mothers received opioid analgesics during labor should be observed closely for signs of respiratory depression.
8.3 Nursing Mothers
Low concentrations of hydromorphone have been detected in human milk in clinical trials. Withdrawal symptoms can occur in breastfeeding infants when maternal administration of an opioid analgesic is stopped. Nursing should not be undertaken while a patient is receiving EXALGO since hydromorphone is excreted in the milk.
8.4 Pediatric Use
The safety and effectiveness of EXALGO in pediatric patients 17 years of age and younger have not been established.
8.5 Geriatric Use
Elderly patients have been shown to be more sensitive to the adverse effects of EXALGO compared to the younger population. Therefore, use extra caution when prescribing EXALGO in elderly patients and reduce the initial dose.
8.6 Neonatal Withdrawal Syndrome
Chronic maternal use of opiates or opioids during pregnancy coexposes the fetus. The newborn may experience subsequent neonatal withdrawal syndrome (NWS). Manifestations of NWS include irritability, hyperactivity, abnormal sleep pattern, high-pitched cry, tremor, vomiting, diarrhea, weight loss, and failure to gain weight. The onset, duration, and severity of the disorder differ based on such factors as the addictive drug used, time and amount of mother’s last dose, and rate of elimination of the drug from the newborn. Approaches to the treatment of this syndrome have included supportive care and, when indicated, drugs such as paregoric or phenobarbital.
8.7 Hepatic Impairment
In a study that used a single 4 mg oral dose of immediate-release hydromorphone tablets, four-fold increases in plasma levels of hydromorphone (Cmax and AUC0-∝) were observed in patients with moderate hepatic impairment (Child-Pugh Group B). Start patients with moderate hepatic impairment on a reduced dose and closely monitored during dose titration. The pharmacokinetics of hydromorphone in severe hepatic impairment patients have not been studied. Further increase in Cmax and AUC0-∝ of hydromorphone in this group is expected, therefore, use an even more conservative starting dose [see Dosage and Administration (2.4)].
8.8 Renal Impairment
Renal impairment affected the pharmacokinetics of hydromorphone and its metabolites following administration of a single 4 mg dose of immediate-release tablets. The effects of renal impairment on hydromorphone pharmacokinetics were two-fold and four-fold increases in plasma levels of hydromorphone (Cmax and AUC0-48h) in moderate (CLcr = 40 to 60 mL/min) and severe (CLcr < 30 mL/min) impairment, respectively. In addition, in patients with severe renal impairment hydromorphone appeared to be more slowly eliminated with longer terminal elimination half-life (40 hours) compared to subjects with normal renal function (15 hours). Start patients with moderate renal impairment on a reduced dose and closely monitor during dose titration. As EXALGO is only intended for once daily administration, consider use of an alternate analgesic that may permit more flexibility with the dosing interval in patients with severe renal impairment [see Dosage and Administration (2.4)].
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
EXALGO contains hydromorphone, a Schedule II controlled substance with a high potential for abuse similar to fentanyl, methadone, morphine, oxycodone, and oxymorphone. EXALGO can be abused and is subject to misuse, abuse, addiction, and criminal diversion [see Warnings and Precautions (5.2)]. The high drug content in the extended release formulation adds to the risk of adverse outcomes from abuse.
9.2 Abuse
All patients treated with opioids, including EXALGO, require careful monitoring for signs of abuse and addiction, because use of opioid analgesic products carries the risk of addiction even under appropriate medical use.
Addiction is a primary, chronic, neurobiologic disease, with genetic, psychosocial, and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving.
"Drug-seeking" behavior is very common to addicts and drug abusers. Drug-seeking tactics include emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing or referral, repeated claims of loss of prescriptions, tampering with prescriptions and reluctance to provide prior medical records or contact information for other treating physician(s). “Doctor shopping” (visiting multiple prescribers) to obtain additional prescriptions is common among drug abusers, people suffering from untreated addiction and criminals seeking drugs to sell.
Abuse and addiction are separate and distinct from physical dependence and tolerance. Physicians should be aware that addiction may not be accompanied by concurrent tolerance and symptoms of physical dependence in all addicts. In addition, abuse of opioids can occur in the absence of true addiction and is characterized by misuse for non-medical purposes, often in combination with other psychoactive substances. Since EXALGO may be diverted for non-medical use, careful record-keeping of prescribing information, including quantity, frequency, and renewal requests is strongly advised.
Proper assessment of the patient, proper prescribing practices, periodic re-evaluation of therapy, and proper dispensing and storage are appropriate measures that help to limit abuse of opioid drugs.
EXALGO is intended for oral use only. Misuse or abuse by breaking, crushing, chewing, or dissolving EXALGO poses a hazard of overdose and death. This risk is increased with concurrent abuse of EXALGO with alcohol and other substances.
With intravenous abuse, the tablet excipients, especially polyethylene oxide, can be expected to result in necrosis and inflammation of cardiac tissues. In addition, parenteral drug abuse is commonly associated with transmission of infectious disease such as hepatitis and HIV.
Healthcare professionals should contact their State Professional Licensing Board or State Controlled Substances Authority for information on how to prevent and detect abuse or diversion of this product.
9.3 Dependence
Tolerance is a state of adaptation in which exposure to a drug induces changes that result in a diminution of one or more of the drug’s effects over time. Tolerance could occur to both the desired and undesired effects of drugs, and may develop at different rates for different effects.
Physical dependence is a state of adaptation that is manifested by an opioid specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist. The opioid abstinence or withdrawal syndrome is characterized by some or all of the following: restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, piloerection, myalgia, mydriasis, irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, increased blood pressure, respiratory rate, or heart rate.
Infants born to mothers physically dependent on opioids will also be physically dependent and may exhibit respiratory difficulties and withdrawal symptoms [see Use in Specific Populations (8.1, 8.2)].
10 OVERDOSAGE
10.1 Symptoms
Acute overdosage with opioids can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, and sometimes bradycardia, hypotension and death. The extended release characteristics of EXALGO should also be taken into account when treating the overdose. Even in the face of improvement, continued medical monitoring is required because of the possibility of extended effects. Deaths due to overdose could occur with abuse and misuse of EXALGO.
Due to the delayed mean apparent peak plasma level of EXALGO occurring at 16 hours following administration as well as the 11 hour mean elimination half-life of EXALGO, patients who receive an overdose will require an extended period of monitoring and treatment that may go beyond 24 to 48 hours.
10.2 Treatment
Give primary attention to the re-establishment of a patent airway and institution of assisted or controlled ventilation. Employ supportive measures (including oxygen and vasopressors) in the management of circulatory shock and pulmonary edema accompanying overdose as indicated. Cardiac arrest or arrhythmias will require advanced life support techniques.
The pure opioid antagonists, such as naloxone and naltrexone are specific antidotes to respiratory depression from opioid overdose. Since the duration of reversal would be expected to be less than the duration of action of hydromorphone in EXALGO, the patient must be carefully monitored until spontaneous respiration is reliably re-established. EXALGO will continue to release and add to the hydromorphone load for up to 24 hours after administration and the management of an overdose should be monitored accordingly, at least 24 to 48 hours beyond the overdose.
Only administer opioid antagonists in the presence of clinically significant respiratory or circulatory depression secondary to hydromorphone overdose. In patients who are physically dependent on any opioid agonist including EXALGO, an abrupt or complete reversal of opioid effects may precipitate an acute abstinence syndrome. The severity of the withdrawal syndrome produced will depend on the degree of physical dependence and the dose of the antagonist administered. Please see the prescribing information for the specific opioid antagonist for details of their proper use.
11 DESCRIPTION
EXALGO tablets contain hydromorphone hydrochloride, a mu-opioid agonist.
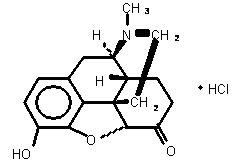
Hydromorphone hydrochloride USP is 4,5α-epoxy-3-hydroxy-17-methlymorphinan-6-one hydrochloride. Hydromorphone hydrochloride is a white or almost white crystalline powder that is freely soluble in water, very slightly soluble in ethanol (96%), and practically insoluble in methylene chloride. Its empirical formula is C17H19NO3•HCl. The compound has the following structural formula:
EXALGO also contains the following inactive ingredients: butylated hydroxytoluene, cellulose acetate, iron oxide black, ferric oxide red (8 mg only), ferric oxide yellow (12 mg and 16 mg only), hypromellose, lactose anhydrous, lactose monohydrate, magnesium stearate, polyethylene glycol, polyethylene oxide, povidone, sodium chloride, titanium dioxide, and triacetin.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Hydromorphone, a semi-synthetic morphine derivative, is a hydrogenated ketone of morphine. Hydromorphone is principally an agonist of mu-receptors, showing a weak affinity for k-receptors. Comparing relative binding affinity for mu- and k-opioid receptors, hydromorphone binds more specifically to mu-receptors than structurally related morphine. As an opioid agonist, the principle therapeutic action of hydromorphone is analgesia. The precise mechanism of action of opioid analgesics is not known but the effects are thought to be mediated through opioid-specific receptors located predominantly in the central nervous system (CNS). Interaction with the mu-opioid receptor subtype is believed to be responsible for most of hydromorphone’s clinical effects. There is no intrinsic limit to the analgesic effect of hydromorphone. Clinically, however, dosage limitations are imposed by the adverse effects, primarily respiratory depression, sedation, nausea, and vomiting, which can result from high doses.
12.2 Pharmacodynamics
Hydromorphone exerts its principal pharmacological effects on the CNS and smooth muscle, including the gastrointestinal tract. These effects are expressed and modulated by binding to specific opioid receptors. Hydromorphone is principally an agonist of mu-receptors, showing a weak affinity for k-receptors. Analgesia occurs as a consequence of the binding of hydromorphone to the mu-receptors of the CNS. Although estimates vary (from 2 to 10 times), oral hydromorphone appears to be approximately 5 times as potent (by weight) as morphine. Respiratory depression occurs principally by direct action on the cerebral respiratory control centers. Hydromorphone may cause nausea and vomiting due to direct stimulation of the chemoreceptor for emesis in the posterior area of the medulla.
Central Nervous System
Hydromorphone produces dose-related respiratory depression by direct action on brain stem respiratory centers. The respiratory depression involves a reduction in the responsiveness of the brain stem respiratory centers to increases in carbon dioxide tension and to electrical stimulation.
Hydromorphone depresses the cough reflex by direct effect on the cough center in the medulla.
Hydromorphone causes miosis, even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomic. Marked mydriasis, rather than miosis, may be seen due to severe hypoxia in overdose situations.
Gastrointestinal Tract and Other Smooth Muscle
Hydromorphone decreases gastric, biliary and pancreatic secretions, and causes a reduction in motility associated with an increase in smooth muscle tone in the antrum of the stomach and duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone may be increased to the point of spasm resulting in constipation. Hydromorphone also can cause an increase in bilary tract pressure as a result of spasm of the sphincter of Oddi.
Cardiovascular System
Hydromorphone may produce peripheral vasodilation which may result in orthostatic hypotension. Release of histamine may occur with or without associated peripheral vasodilation. Manifestations of histamine release and/or peripheral vasodilation may include pruritus, flushing, red eyes, and sweating.
Endocrine System
Opioid agonists have been shown to have a variety of effects on the secretion of hormones. Opioids inhibit the secretion of adrenocorticotropic hormone (ACTH), cortisol, and luteinizing hormone (LH) in humans. They also stimulate prolactin, growth hormone (GH) secretion, and pancreatic secretion of insulin and glucagon in humans and other species, rats and dogs. Thyroid stimulating hormone (TSH) has been shown to be both inhibited and stimulated by opioids.
12.3 Pharmacokinetics
Absorption
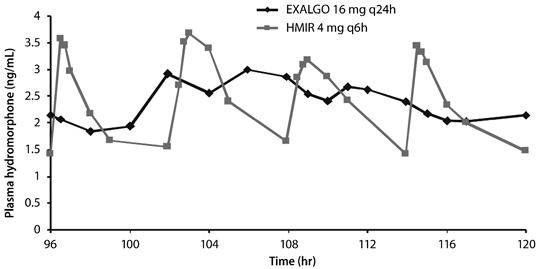
EXALGO is an extended release formulation of hydromorphone that produces a gradual increase in hydromorphone concentrations. Following a single dose administration of EXALGO, plasma concentrations gradually increase over 6 to 8 hours, and thereafter concentrations are sustained for approximately 18 to 24 hours post-dose. The median Tmax values ranged from 12 to 16 hours. The mean half-life was approximately 11 hours, ranging from 8 to 15 hours in most individual subjects. Linear pharmacokinetics has been demonstrated for EXALGO over the dose range 8 to 64 mg, with a dose-proportional increase in Cmax and overall exposure (AUC0-∝) (see Table 3). Steady-state plasma concentrations are approximately twice those observed following the first dose, and steady state is reached after 3 to 4 days of once-daily dosing of EXALGO. At steady state, EXALGO given once daily maintained hydromorphone plasma concentrations within the same concentration range as the immediate-release tablet given 4 times daily at the same total daily dose, and diminished the fluctuations between peak and trough concentrations seen with the immediate-release tablet (see Figure 1). The bioavailability of EXALGO once daily and immediate-release hydromorphone four times daily in adults is comparable as presented in Table 4.
| Regimen | Dosage | Tmax* (hrs) | Cmax(ng/mL) | AUC (ng•hr/mL) | T½ (hr) |
|---|---|---|---|---|---|
| NA = not applicable | |||||
| Single Dose (N = 31) | 8 mg | 12 (4-30) | 0.93 (1.01) | 18.1 (5.8) | 10.6 (4.3) |
| 16 mg | 16 (6-30) | 1.69 (0.78) | 36.5 (11.3) | 10.3 (2.4) | |
| 32 mg | 16 (4-24) | 3.25 (1.37) | 72.2 (24.3) | 11.0 (3.2) | |
| 64 mg | 16 (6-30) | 6.61 (1.75) | 156.0 (30.6) | 10.9 (3.8) | |
| Multiple Dose† (N = 29) | 16 mg q24h | 12 (6-24) | 3.54 (0.96)‡ | 57.6 (16.3) | NA |
| IR 4 mg q6h | 0.75 (0.5-2) | 5.28 (1.37)§ | 54.8 (14.8) | NA | |
Food Effect
The pharmacokinetics of EXALGO are not affected by food as indicated by bioequivalence when administered under fed and fasting conditions. Therefore, EXALGO may be administered without regard to meals. When a 16 mg dose of EXALGO was administered to healthy volunteers immediately following a high-fat meal, the median time to Cmax (Tmax) was minimally affected by the high-fat meal occurring at 16 hours compared to 18 hours while fasting.
Alcohol Effect
An in vivo study examined the effect of alcohol (40%, 20%, 4% and 0%) on the bioavailability of a single dose of 16 mg of EXALGO in healthy, fasted or fed volunteers. The results showed that the hydromorphone mean AUC0-∝ was 5% higher and 4% lower (not statistically significant) in the fasted and fed groups respectively after co-administration of 240 mL of 40% alcohol. The AUC0-∝ was similarly unaffected in subjects following the co-administration of EXALGO and alcohol (240 mL of 20% or 4% alcohol).
The change in geometric mean Cmax with concomitant administration of alcohol and EXALGO ranged from an increase of 10% to 31% across all conditions studied. The change in mean Cmax was greater in the fasted group of subjects. Following concomitant administration of 240 mL of 40% alcohol while fasting the mean Cmax increased by 37%, and up to 151% in an individual subject. Following the concomitant administration of 240 mL of 20% alcohol while fasting the mean Cmax increased by 35% and up to 139% in an individual subject. Following the concomitant administration of 240 mL of 4% alcohol while fasting the mean Cmax increased by 19% on average and as much as 73% for an individual subject. The range of median Tmax for the fed and fasted treatments with 4%, 20% and 40% alcohol was 12 to 16 hours compared to 16 hours for the 0% alcohol treatments.
Distribution
Following intravenous administration of hydromorphone to healthy volunteers, the mean volume of distribution was 2.9 (±1.3) L/kg, suggesting extensive tissue distribution. The mean extent of binding of hydromorphone to human plasma proteins was determined to be 27% in an in vitro study.
Metabolism
After oral administration of an immediate-release formulation, hydromorphone undergoes extensive first-pass metabolism and is metabolized primarily in the liver by glucuronidation to hydromorphone-3-glucuronide, which follows a similar time course to hydromorphone in plasma. Exposure to the glucuronide metabolite is 35 to 40 times higher than exposure to the parent drug.
Excretion
Approximately 75% of the administered dose is excreted in urine. Most of the administered hydromorphone dose is excreted as metabolites. Approximately 7% and 1% of the dose are excreted as unchanged hydromorphone in urine and feces, respectively.
Special Populations
Geriatric - Age has no effect on the pharmacokinetics of hydromorphone based on the data obtained from an immediate release product.
Gender - Females appeared to have approximately 10% higher mean systemic exposure in terms of Cmax and AUC values.
Race - The effect of race on EXALGO pharmacokinetics has not been studied.
Pediatric - Pharmacokinetics of EXALGO were not evaluated in the pediatric population.
Hepatic Impairment - In a study that used a single 4 mg oral dose of immediate-release hydromorphone tablets, four-fold increases in plasma levels of hydromorphone (Cmax and AUC0-∝) were observed in patients with moderate hepatic impairment (Child-Pugh Group B). Pharmacokinetics of hydromorphone in severe hepatic impairment patients has not been studied. Further increase in Cmax and AUC0-∝ of hydromorphone in this group is expected. Start patients with moderate and severe hepatic impairment on a reduced dose of EXALGO and closely monitor during dose titration [see Dosage and Administration (2.4) and Specific Populations (8.7)].
Renal Impairment - Renal impairment affected the pharmacokinetics of hydromorphone and its metabolites following administration of a single 4 mg dose of immediate-release tablets. The effects of renal impairment on hydromorphone pharmacokinetics were two-fold and four-fold increases in plasma levels of hydromorphone (Cmax and AUC0-48h) in moderate (CLcr = 40 to 60 mL/min) and severe (CLcr < 30 mL/min) impairment, respectively. In addition, in patients with severe renal impairment hydromorphone appeared to be more slowly eliminated with longer terminal elimination half-life (40 hr) compared to subjects with normal renal function (15 hr). Start patients with moderate renal impairment on a reduced dose of EXALGO and closely monitored during dose titration. As EXALGO is only intended for once-daily administration, consider use of an alternate analgesic that may permit more flexibility with the dosing interval in patients with severe renal impairment [see Dosage and Administration (2.4) and Use in Specific Populations (8.8)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term studies in animals to evaluate the carcinogenic potential of hydromorphone hydrochloride have not been conducted.
Mutagenesis
Hydromorphone was not mutagenic in the in vitro bacterial reverse mutation assay (Ames assay). Hydromorphone was not clastogenic in either the in vitro human lymphocyte chromosome aberration assay or the in vivo mouse micronucleus assay.
Impairment of Fertility
Hydromorphone given orally to rats during the mating period caused a slight but statistically significant reduction in implantations at 6.25 mg/kg/day (~1.2 times the human exposure following to 32 mg/day).
14 CLINICAL STUDIES
EXALGO was investigated in a double-blind, placebo-controlled, randomized withdrawal study in opioid tolerant patients with moderate-to-severe low back pain. Patients were considered opioid tolerant if they were currently on opioid therapy that was ≥60 mg/day of oral morphine equivalent for at least 2 months prior to screening. Patients entered an open-label conversion and titration phase with EXALGO, were converted to a starting dose that was approximately 75% of their total daily morphine equivalent dose, and were dosed once daily until adequate pain control was achieved while exhibiting tolerable side effects. Supplemental immediate-release hydromorphone tablets were allowed throughout the study. Patients who achieved a stable dose entered a 12-week, double-blind, placebo-controlled, randomized treatment phase. Mean daily dose at randomization was 37.8 mg/day (range of 12 mg/day to 64 mg/day). Fifty-eight (58) percent of patients were successfully titrated to a stable dose of EXALGO during the open-label conversion and titration phase.
During the double-blind treatment phase, patients randomized to EXALGO continued with the stable dose achieved in the conversion and titration phase of the study. Patients randomized to placebo received, in a blinded manner, EXALGO and matching placebo in doses tapering from the stable dose achieved in conversion and titration. During the taper down period, patients were allowed immediate-release hydromorphone tablets as supplemental analgesia to minimize opioid withdrawal symptoms in placebo patients. After the taper period, the number of immediate-release hydromorphone tablets was limited to two tablets per day. Forty-nine (49) percent of patients treated with EXALGO and 33% of patients treated with placebo completed the 12-week treatment period.
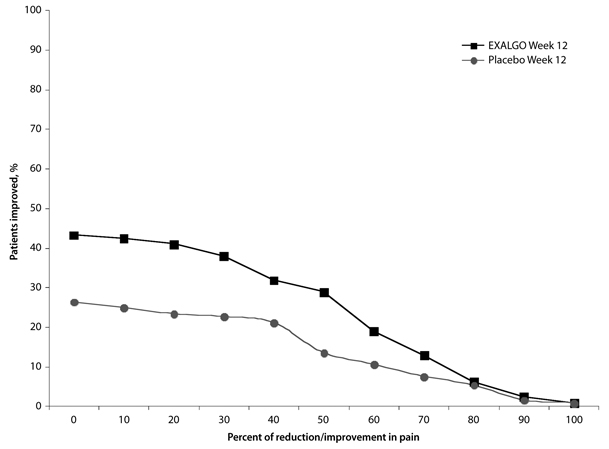
EXALGO provided superior analgesia compared to placebo. There was a significant difference between the mean changes from Baseline to Week 12 or Final Visit in average weekly pain intensity Numeric Rating Scale (NRS) scores obtained from patient diaries between the two groups. The proportion of patients with various degrees of improvement from screening to Week 12 or Final Visit is shown in Figure 2. For this analysis, patients who discontinued treatment for any reason prior to Week 12 were assigned a value of zero improvement.
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 Storage and Handling
EXALGO contains hydromorphone which is a controlled substance. Like fentanyl, methadone, morphine, oxycodone, and oxymorphone, hydromorphone is controlled under Schedule II of the Federal Controlled Substances Act. EXALGO may be targeted for theft and diversion by criminals [see Warnings and Precautions (5)]. For further information on this product, healthcare professionals can contact a medical information specialist at Covidien at 1-800-778-7898.
Store at 25ºC (77ºF); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature].
16.2 Disposal of EXALGO
Patients and members of their household must be advised to dispose of any tablets remaining from a prescription as soon as they are no longer needed. Information is available in the Medication Guide. To dispose of unused EXALGO flush all remaining tablets down the toilet.
16.3 How Supplied
| Strength | Color | Tablet Description | Bottle Count | NDC |
|---|---|---|---|---|
| 8 mg | Red | Round, biconvex, printed with “EXH 8” | 100 | 23635-408-01 |
| 12 mg | Dark yellow | Round, biconvex, printed with “EXH 12” | 100 | 23635-412-01 |
| 16 mg | Yellow | Round, biconvex, printed with “EXH 16” | 100 | 23635-416-01 |
17 PATIENT COUNSELING INFORMATION
17.1 Safe Use
Before initiating treatment with EXALGO, explain the points listed below to caregivers and patients. Instruct patients to read the Medication Guide each time EXALGO is dispensed because new information may be available.
- Advise patients that EXALGO is for use only in patients who are already receiving opioid pain medicine and their body is used to taking these medications.
- Advise patients that EXALGO contains hydromorphone, and should be taken only as directed.
- Advise patients that EXALGO must be swallowed whole. The extended release tablets may release all their contents at once if broken, chewed, crushed, or dissolved resulting in risk of fatal overdose of hydromorphone.
- Advise patients not to take another dose of EXALGO within 24 hours.
- Advise patients never to change the dose of EXALGO without first consulting their healthcare provider. Individualization of dosage is essential to make optimal use of this medication. Advise patients regarding the need to contact their healthcare provider if pain control is inadequate and to report episodes of breakthrough pain and adverse experiences occurring during therapy to their healthcare provider.
- Advise patients to keep EXALGO tablets in a secure place out of the reach of children. Accidental consumption of EXALGO, especially in children, can result in a fatal overdose of hydromorphone.
- Advise patients that EXALGO must not be given to anyone other than the individual for whom it was prescribed. Instruct patients against use by individuals other than the patient for whom it was prescribed, as such inappropriate use may have severe medical consequences, including death.
17.2 Risk of Gastrointestinal Obstruction
Advise patients that people with certain stomach or intestinal problems such as narrowing of the intestines or previous surgery may be at higher risk of developing a blockage. Symptoms include abdominal distension, abdominal pain, severe constipation, or vomiting. Instruct patients to contact their healthcare provider immediately if they develop these symptoms.
17.3 Mental and/or Physical Ability
Caution patients that EXALGO may cause drowsiness, dizziness, or lightheadedness, and may impair mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a car or operating machinery.
17.4 Avoidance of Alcohol or Other CNS Depressants
Advise patients not to combine EXALGO with other pain medications, sleep aids, or tranquilizers except by the orders of a healthcare professional, and not to consume alcohol while taking EXALGO, because dangerous additive effects could occur, resulting in serious injury or death.
17.5 Pregnancy
Advise women of childbearing potential who become, or who plan to become, pregnant to consult their healthcare professional regarding the effects of EXALGO and other drug use during pregnancy on themselves and their unborn child. Advise women that EXALGO can pass through breast milk and may cause serious harm to a nursing infant so they should not breastfeed while taking EXALGO.
17.6 Cessation of Therapy
Advise patients that if they have been receiving treatment with EXALGO for more than a few days to weeks and the medicine is no longer needed, it is important to taper the dose and that abruptly discontinuing the medication could cause withdrawal symptoms. Provide patients with a dose schedule to accomplish a gradual discontinuation of the medication.
Advise patients that if they stop taking this medication for 3 or more days, to contact their healthcare professional before restarting their medication, to avoid complications of being opioid non-tolerant.
17.7 Drug of Abuse
Advise patients that the active ingredient in EXALGO is hydromorphone, which is a drug that some people abuse. EXALGO must be taken only by the patient for whom it was prescribed, and it must be protected from theft or misuse in the work or home environment.
17.8 Constipation
Advise patients that taking EXALGO has the potential to cause severe constipation. Consider the use of appropriate laxatives and/or stool softeners and other therapeutic approaches with the initiation of EXALGO therapy.
Advise patients that the drug is contained within a non-absorbable shell designed to release the drug at a controlled rate. The tablet shell is eliminated from the body and patients should not be alarmed if they occasionally notice something that looks like a tablet in their stool.
17.9 Storage/Destruction of Unused Tablets
Advise patients that when EXALGO is no longer needed to flush the unused tablets down the toilet.
17.10 FDA-Approved Patient Labeling
MEDICATION GUIDE
EXALGO™ (eks-al-goh)
(hydromorphone HCl) Extended-Release Tablets CII
|
IMPORTANT: |
Read the Medication Guide that comes with EXALGO before you start taking it and each time you get a new prescription. There may be new information. This Medication Guide does not take the place of talking to your healthcare provider about your medical condition or your treatment. Share this important information with members of your household.
What is the most important information I should know about EXALGO?
1. EXALGO overdose can cause life threatening breathing problems that can lead to death.
- Do not take EXALGO unless you are already regularly using opioid pain medicines around-the-clock and your body is used to taking these medicines. This means you are opioid tolerant.
- Take EXALGO exactly as prescribed by your healthcare provider. Do not take more than your prescribed daily dose. It is important that you do not take another dose of EXALGO within 24 hours.
- Swallow the EXALGO tablet whole. Do not break, chew, crush, or dissolve EXALGO before swallowing, or inject the contents. You could receive the full daily dose of medicine too fast. This is very dangerous. It may cause you to have trouble breathing, and lead to death. If you cannot swallow EXALGO whole, tell your healthcare provider. You will need a different pain medicine.
- EXALGO is not for use to treat pain that you only have once in a while (“as-needed”).
- EXALGO is not for use for short-term pain relief from injuries or surgery.
- It is important for you to stay under the care of your healthcare provider while taking EXALGO.
2. Prevent theft, misuse, or abuse. Keep EXALGO in a safe place to protect it from being stolen. EXALGO can be a target for people who misuse or abuse prescription medicines or street drugs.
3. Never give EXALGO to anyone else, even if they have the same symptoms you have. It may harm them or cause death. Selling or giving away this medicine is against the law.
What is EXALGO?
- EXALGO is a prescription medicine that contains the opioid (narcotic) pain medicine hydromorphone. The medicine in EXALGO is slowly released over 24 hours. If you break, chew, crush, or dissolve EXALGO before swallowing, or inject the contents, the hydromorphone hydrochloride may be released too fast and you may overdose (see What is the most important information I should know about EXALGO?).
- EXALGO is a strong opioid pain medicine. EXALGO is used in people who are opioid tolerant, to manage moderate to severe pain that continues around-the-clock and is expected to last for a long period of time.
- EXALGO is a federally controlled substance (CII) because it contains a strong opioid pain medicine that can be a target for people who abuse prescription medicines or street drugs.
Who should not take EXALGO?
Do not take EXALGO if you:
- are not already regularly taking opioid pain medicine and your body is not used to taking these medicines for your pain. This means you are not opioid tolerant.
- are having an asthma attack or have severe asthma, trouble breathing, or certain other lung problems.
- have a bowel blockage called paralytic ileus.
- have narrowing of the stomach or intestines, or have had surgery to your stomach or intestines.
- are allergic to any of the ingredients in EXALGO or to medicines that contain sulfite. See the end of this Medication Guide for a complete list of the ingredients in EXALGO.
Talk to your healthcare provider before taking this medicine if you have any of the conditions listed above.
What should I tell my healthcare provider before starting EXALGO?
EXALGO may not be right for you. Before taking EXALGO, tell your healthcare provider if you:
- have trouble breathing or lung problems such as asthma, wheezing, or shortness of breath.
- have had a head injury or brain problem.
- have liver or kidney problems.
- have an adrenal gland problem, such as Addison’s disease.
- have thyroid problems.
- have seizures (convulsions or fits).
- have problems with your pancreas or gallbladder.
- have constipation.
- have had stomach or intestinal surgery, or a blockage in your stomach or intestine.
- have prostate enlargement or problems urinating.
- have low blood pressure.
- have mental problems including depression, anxiety, or hallucinations (seeing or hearing things that are not there).
- have or had a drinking problem or alcoholism, or a family history of this problem.
- have or had a drug abuse or addiction problem in the past, or a family history of this problem.
- have any other medical conditions.
-
are pregnant or planning to become pregnant. If you take EXALGO regularly before your baby is born, your newborn baby may have withdrawal symptoms because their body has become used to the medicine. Symptoms of withdrawal in a newborn baby may include:
- irritability
- crying more than usual
- shaking (tremors)
- jitteriness
- breathing faster than normal
- diarrhea or more stools than normal
- vomiting
- fever
- are breastfeeding. Do not breastfeed while taking EXALGO. Some EXALGO passes into breast milk. A nursing baby could become drowsy or have difficulty breathing or feeding well. If you stop breastfeeding or suddenly stop taking EXALGO while breastfeeding, your baby may have withdrawal symptoms. See the list of withdrawal symptoms above.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Some medicines may cause serious or life-threatening medical problems when taken with EXALGO. Sometimes, the doses of certain medicines and EXALGO need to be changed if used together. Be especially careful about other medicines that make you sleepy such as:
- other pain medicines
- antidepressant medicines
- sleeping pills
- antihistamines
- anti-anxiety medicines
- muscle relaxants
- anti-nausea medicines
- tranquilizers
Do not take EXALGO if you already take a monoamine oxidase (MAO) inhibitor medicine or within 14 days after you stop taking an MAO inhibitor medicine.
Do not take any new medicine while using EXALGO until you have talked to your healthcare provider or pharmacist. They will tell you if it is safe to take other medicines while you are taking EXALGO. Ask your healthcare provider if you are not sure if your medicine is one listed above.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take EXALGO?
- Take EXALGO exactly as prescribed by your healthcare provider. Do not change your dose unless your healthcare provider tells you to.
- Take EXALGO one time each day at the same time every day.
- Swallow the EXALGO tablet whole. Do not break, chew, crush, or dissolve EXALGO before swallowing, or inject the contents of EXALGO (see What is the most important information I should know about EXALGO?). Tell your healthcare provider if you cannot swallow EXALGO whole. You will need to take a different pain medicine.
- EXALGO can be taken with or without food.
- The EXALGO tablet is contained in a hard shell that does not dissolve in your body. The tablet shell passes through your body in your stool. You may notice something that looks like a tablet in your bowel movement. This is normal.
- Call your healthcare provider if the dose of EXALGO that you are taking does not control your pain.
- If you stop taking EXALGO for 3 or more days, call your healthcare provider before restarting the medicine.
- If you are not sure if you have taken your dose, do not take another dose. Taking more EXALGO than prescribed may cause you to overdose. If you are not sure what to do, call your healthcare provider.
- If you take too much EXALGO or overdose, call 911 or get emergency help right away.
- Do not stop taking EXALGO without talking to your healthcare provider. Opioid medicines such as EXALGO can cause physical dependence. You should not suddenly stop taking EXALGO because you may become sick with uncomfortable withdrawal symptoms. If your healthcare provider decides you no longer need EXALGO, ask how to slowly stop taking this medicine to avoid uncomfortable withdrawal symptoms.
What should I avoid while taking EXALGO?
- Do not drive or operate heavy machinery, or do other dangerous activities, until you know how EXALGO affects how alert you are. EXALGO can make you sleepy, and cause you to feel dizzy or lightheaded. This may affect your ability to think and react. Ask your healthcare provider when it is okay to do these activities.
- Do not drink alcohol or use prescription or non-prescription medicines that contain alcohol while taking EXALGO. Using alcohol while taking EXALGO may cause you to overdose and die.
What are the possible side effects of EXALGO?
EXALGO can cause serious side effects that can lead to death (see What is the most important information I should know about EXALGO?).
Call your healthcare provider or get emergency medical help if you:
- have trouble breathing.
- have extreme drowsiness with slowed breathing.
- have shallow breathing (little chest movement with breathing).
- feel faint, dizzy, confused, or have other unusual symptoms.
These can be symptoms that you have taken too much (overdose) EXALGO or the dose is too high for you. These symptoms may lead to serious problems or death if not treated right away.
Drop in your blood pressure. This can make you feel dizzy if you get up too fast from sitting or lying down. Low blood pressure is also more likely to happen if you take other medicines that can also lower your blood pressure. Severe low blood pressure can happen if you lose blood or take certain other medicines.
Symptoms of stomach or intestinal blockage. EXALGO tablets do not change shape when they pass through your stomach and intestine. People who have certain stomach or intestinal problems, such as narrowing (stricture), or who have had surgery in these areas may get symptoms of a blockage. Tell your healthcare provider right away if you get any of these symptoms:
- vomiting
- severe constipation
- abdominal pain
- abdominal distension
Allergic reactions. EXALGO contains sodium metabisulfite, a sulfite that may cause allergic-type reactions. Symptoms of an allergic reaction to EXALGO and sulfites may include:
- feel dizzy or faint
- trouble breathing
- pounding heart beat
- chest pain
- swelling of the face, throat, or tongue
- feeling of doom
Physical dependence. Stopping EXALGO suddenly can make you sick with withdrawal symptoms because your body has become used to it. Talk to your healthcare provider about slowly stopping EXALGO. Physical dependence is not the same thing as addiction. Tell your healthcare provider if you have any of the following symptoms of withdrawal while slowly stopping EXALGO:
- feel restless
- tearing eyes
- runny nose
- yawning
- sweating
- chills or hair on your arms “standing up”
- muscle aches, backache, joint pain
- weakness
- dilated pupils of your eyes
- feel irritable or anxious
- nausea, loss of appetite, vomiting, diarrhea
- increase in your blood pressure, breathing faster, or your heart beats faster
- sleep problems
There is a chance of abuse or addiction with EXALGO. The chance is higher if you are, or have been addicted to or abused other medicines, street drugs, or alcohol, or if you have a history of mental problems.
The most common side effects of EXALGO include:
- constipation
- nausea
- drowsiness or sleepiness
- headache
- vomiting
- tiredness
- dizziness
Constipation (not enough or hard bowel movements) is a common side effect of pain medicines (opioids) including EXALGO and is unlikely to go away without treatment. Talk to your healthcare provider about dietary changes, the use of laxatives (medicines to treat constipation) and stool softeners to prevent or treat constipation while taking EXALGO.
These are not all the possible side effects of EXALGO. Talk to your healthcare provider if you have any side effects that bother you or do not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store EXALGO?
- Store EXALGO at 59° to 86°F (15° to 30°C).
- Keep EXALGO in the container it comes in.
- Keep EXALGO dry and away from heat.
- After you stop taking EXALGO flush any unused tablets down the toilet.
Keep EXALGO in a safe and secure place away from children.
General information about EXALGO
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use EXALGO for a purpose for which it was not prescribed. Do not give EXALGO to other people, even if they have the same symptoms you have. EXALGO can harm other people and even cause death. Sharing EXALGO is against the law.
This Medication Guide summarizes the most important information about EXALGO. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about EXALGO that is written for healthcare professionals.
For further information or to report suspected adverse reactions, go to www.exalgo.com or contact Covidien at 1-800-778-7898.
What are the ingredients of EXALGO?
- Active Ingredient: hydromorphone hydrochloride.
- Inactive Ingredients: butylated hydroxytoluene, cellulose acetate, iron oxide black, hypromellose, lactose anhydrous, lactose monohydrate, magnesium stearate, polyethylene glycol, polyethylene oxide, povidone, sodium chloride, titanium dioxide and triacetin. The 8 mg tablets also contain ferric oxide red. The 12 and 16 mg tablets also contain ferric oxide yellow.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
OROS is a registered trademark of ALZA Corporation.
EXALGO is a trademark of Mallinckrodt Inc.
COVIDIEN, COVIDIEN with logo and Covidien logo are U.S. and/or internationally registered trademarks of Covidien AG.
© 2010 Mallinckrodt Inc., a Covidien company
Distributed by:
Mallinckrodt Brand Pharmaceuticals, Inc.
Hazelwood, MO 63042 USA
Issued 03/2010-B
10206401
Mallinckrodt
COVIDIEN™
PRINCIPAL DISPLAY PANEL - 8 mg
NDC 23635-408-01
100 TABLETS
EXALGO™ Once Daily
(hydromorphone HCl)
Extended-Release Tablets
CII
8 mg Rx only
Each tablet contains:
Hydromorphone Hydrochloride USP. . . . . . . 8 mg
For opioid tolerant patients only
Dispense the accompanying Medication
Guide to each patient
Mallinckrodt

PRINCIPAL DISPLAY PANEL - 12 mg
NDC 23635-412-01
100 TABLETS
EXALGO™ Once Daily
(hydromorphone HCl)
Extended-Release Tablets
CII
12 mg Rx only
Each tablet contains:
Hydromorphone Hydrochloride USP. . . . . . . 12 mg
For opioid tolerant patients only
Dispense the accompanying Medication
Guide to each patient
Mallinckrodt

PRINCIPAL DISPLAY PANEL - 16 mg
NDC 23635-416-01
100 TABLETS
EXALGO™ Once Daily
(hydromorphone HCl)
Extended-Release Tablets
CII
16 mg Rx only
Each tablet contains:
Hydromorphone Hydrochloride USP. . . . . . . 16 mg
For opioid tolerant patients only
Dispense the accompanying Medication
Guide to each patient
Mallinckrodt

| EXALGO
hydromorphone hydrochloride tablet, extended release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA021217 | 04/06/2010 | |
| EXALGO
hydromorphone hydrochloride tablet, extended release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA021217 | 04/06/2010 | |
| EXALGO
hydromorphone hydrochloride tablet, extended release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA021217 | 04/06/2010 | |
| Labeler - MALLINCKRODT INC (BRAND PHARMACEUTICALS) (047021092) |