DEPOCYT
-
cytarabine
injection, lipid complex
Enzon Pharmaceutical, Inc.
----------
RX ONLYDEPOCYT®
(cytarabine liposome injection)
For Intrathecal Use Only
50 mg vial
WARNING
DepoCyt® (cytarabine liposome injection) should be administered only under the supervision of a qualified physician experienced in the use of intrathecal cancer chemotherapeutic agents. Appropriate management of complications is possible only when adequate diagnostic and treatment facilities are readily available. In all clinical studies, chemical arachnoiditis, a syndrome manifested primarily by nausea, vomiting, headache and fever, was a common adverse event. If left untreated, chemical arachnoiditis may be fatal. The incidence and severity of chemical arachnoiditis can be reduced by coadministration of dexamethasone (see WARNINGS). Patients receiving DepoCyt should be treated concurrently with dexamethasone to mitigate the symptoms of chemical arachnoiditis (see DOSAGE AND ADMINISTRATION).
DESCRIPTION
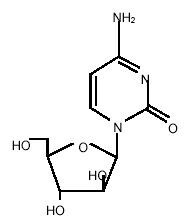
DepoCyt® (cytarabine liposome injection) is a sterile, injectable suspension of the antimetabolite cytarabine, encapsulated into multivesicular lipid-based particles. Chemically, cytarabine is 4-amino-1-β-D-arabinofuranosyl-2(1H)-pyrimidinone, also known as cytosine arabinoside (C9H13N3O5, molecular weight 243.22).
The following is an artist’s rendition of a DepoCyt particle:
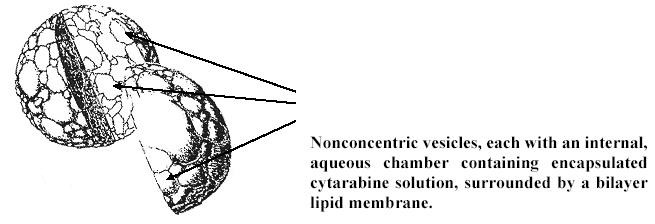
DepoCyt is available in 5 mL, ready-to-use, single-use vials containing 50 mg of cytarabine. DepoCyt is formulated as a sterile, non-pyrogenic, white to off-white suspension of cytarabine in Sodium Chloride 0.9% w/v in Water for Injection. DepoCyt is preservative-free. Cytarabine, the active ingredient, is present at a concentration of 10 mg/mL, and is encapsulated in the particles. Inactive ingredients at their respective approximate concentrations are cholesterol, 4.1 mg/mL; triolein, 1.2 mg/mL; dioleoylphosphatidylcholine (DOPC), 5.7 mg/mL; and dipalmitoylphosphatidylglycerol (DPPG), 1.0 mg/mL. The pH of the product falls within the range from 5.5 to 8.5.
CLINICAL PHARMACOLOGY
Mechanism of Action
DepoCyt® (cytarabine liposome injection) is a sustained-release formulation of the active ingredient cytarabine designed for direct administration into the cerebrospinal fluid (CSF). Cytarabine is a cell cycle phase-specific antineoplastic agent, affecting cells only during the S-phase of cell division. Intracellularly, cytarabine is converted into cytarabine-5’-triphosphate (ara-CTP), which is the active metabolite. The mechanism of action is not completely understood, but it appears that ara-CTP acts primarily through inhibition of DNA polymerase. Incorporation into DNA and RNA may also contribute to cytarabine cytotoxicity. Cytarabine is cytotoxic to a wide variety of proliferating mammalian cells in culture.
Pharmacokinetics
Following intrathecal administration of DepoCyt 50 mg during the induction phase, peak levels of free CSF cytarabine were observed within 1 hour of dosing and ranged from 30 to 50 mcg/mL. The terminal half-life for the free CSF cytarabine ranged from of 5.9 to 82.4 hours. Systemic exposure to cytarabine was negligible following intrathecal administration of DepoCyt 50 mg.
Metabolism and Elimination
The primary route of elimination of cytarabine is metabolism to the inactive compound ara-U, followed by urinary excretion of ara-U. In contrast to systemically administered cytarabine, which is rapidly metabolized to ara-U, conversion to ara-U in the CSF is negligible after intrathecal administration because of the significantly lower cytidine deaminase activity in the CNS tissues and CSF. The CSF clearance rate of cytarabine is similar to the CSF bulk flow rate of 0.24 mL/min.
Drug Interactions
No formal assessments of pharmacokinetic drug-drug interactions between DepoCyt and other agents have been conducted.
Special Populations
The effects of gender or race on the pharmacokinetics of DepoCyt have not been studied, nor has the effect of renal or hepatic impairment.
CLINICAL STUDIES
DepoCyt® (cytarabine liposome injection) was studied in 2 controlled clinical studies that enrolled patients with neoplastic meningitis. The first study, which was a randomized, multi-center, multi-arm study involving a total of 99 treated patients, compared 50 mg of DepoCyt administered every 2 weeks to standard intrathecal chemotherapy administered twice a week to patients with solid tumors, lymphoma, or leukemia. For patients with lymphoma, standard therapy consisted of 50 mg of unencapsulated cytarabine given twice a week. Thirty-three lymphoma patients (17 DepoCyt, 16 cytarabine) were treated. Patients went off study if they had not achieved a complete response defined as clearing of the CSF from all previously positive sites in the absence of progression of neurological symptoms, after 4 weeks of treatment with study drug.
In the first study, complete response was prospectively defined as (1) conversion, confirmed by a blinded central pathologist, from a positive examination of the CSF for malignant cells to a negative examination on two separate occasions (at least 3 days apart, on day 29 and later) at all initially positive sites, together with (2) an absence of neurological progression during the treatment period.
The complete response rates in the first study of lymphoma are shown in Table 1. Although there was a plan for central pathology review of the data, in 4 of the 7 responding patients on the DepoCyt arm this was not accomplished and these cases were considered to have had a complete response based on the reading of an unblinded pathologist. The median overall survival of all treated patients was 99.5 days in the DepoCyt group and 63 days in the cytarabine group. In both groups the majority of patients died from progressive systemic disease, not neoplastic meningitis.
The second study was a randomized, multi-center, multi-arm study involving a total of 124 treated patients with either solid tumors or lymphomas. In this study, 24 patients with lymphoma were randomized and treated with DepoCyt or cytarabine. Patients received 6 two-week induction cycles of DepoCyt 50 mg every 2 weeks or cytarabine 50 mg twice weekly. Patients then received four maintenance cycles of DepoCyt 50 mg every 4 weeks, or cytarabine 50 mg weekly for 4 weeks. In both studies, patients received concurrent treatment with dexamethasone to minimize symptoms associated with chemical arachnoiditis (see WARNINGS and DOSAGE AND ADMINISTRATION). In this study, cytological response was assessed in a blinded fashion utilizing a similar definition as in the first study. The results in patients with lymphomatous meningitis are shown in Table 1.
| DepoCyt® | Cytarabine | |
| Study 1 95% CI | 7/17 (41%) (18%, 67%) | 1/16 (6%) (0%, 30%) |
| Study 2 95% CI | 4/12 (33%) (10%, 65%) | 2/12 (17%) (2%, 48%) |
INDICATIONS
DepoCyt® (cytarabine liposome injection) is indicated for the intrathecal treatment of lymphomatous meningitis.
CONTRAINDICATIONS
DepoCyt® (cytarabine liposome injection) is contraindicated in patients who are hypersensitive to cytarabine or any component of the formulation, and in patients with active meningeal infection.
WARNINGS (see boxed WARNING)
DepoCyt®(cytarabine liposome injection) should be administered only under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents. Appropriate management of complications is possible only when adequate diagnostic and treatment facilities are readily available. Chemical arachnoiditis, a syndrome manifested primarily by nausea, vomiting, headache and fever, has been a common adverse event in all studies. If left untreated, chemical arachnoiditis may be fatal. The incidence and severity of chemical arachnoiditis can be reduced by coadministration of dexamethasone. Patients receiving DepoCyt should be treated concurrently with dexamethasone to mitigate the symptoms of chemical arachnoiditis (see DOSAGE AND ADMINISTRATION). Infectious meningitis may be associated with intrathecal drug administration. Hydrocephalus has also been reported, possibly precipitated by arachnoiditis.
During the clinical studies, 2 deaths related to DepoCyt were reported. One patient died after developing encephalopathy 36 hours after an intraventricular dose of DepoCyt, 125 mg. This patient was receiving concurrent whole-brain irradiation and had previously received systemic chemotherapy with cyclophosphamide, doxorubicin, and fluorouracil, as well as intraventricular methotrexate. The other patient received DepoCyt, 50 mg by the intraventricular route and developed focal seizures progressing to status epilepticus. This patient died approximately 8 weeks after the last dose of study medication. In the controlled lymphoma study, the patient incidence of seizures was higher in the DepoCyt group (4/17, 23.5%) than in the cytarabine group (1/16, 6.3%). The death of 1 additional patient was considered “possibly” related to DepoCyt. He was a 63-year-old with extensive lymphoma involving the nasopharynx, brain, and meninges with multiple neurologic deficits who died of apparent disease progression 4 days after his second dose of DepoCyt.
After intrathecal administration of cytarabine the most frequently reported reactions are nausea, vomiting and fever. Intrathecal administration of cytarabine may cause myelopathy and other neurologic toxicity and can rarely lead to a permanent neurologic deficit. Administration of intrathecal cytarabine in combination with other chemotherapeutic agents or with cranial/spinal irradiation may increase this risk of neurotoxicity.
Blockage to CSF flow may result in increased free cytarabine concentrations in the CSF and an increased risk of neurotoxicity.
Following intrathecal administration of DepoCyt, central nervous system toxicity, including persistent extreme somnolence, hemiplegia, visual disturbances including blindness, deafness and cranial nerve palsies have been reported. Symptoms and signs of peripheral neuropathy, such as pain, numbness, paresthesia, weakness, and impaired bowel and bladder control have also been observed.
Pregnancy Category D
There are no studies assessing the reproductive toxicity of DepoCyt. Cytarabine, the active component of DepoCyt, can cause fetal harm if a pregnant woman is exposed to the drug systemically. Three anecdotal cases of major limb malformations have been reported in infants after their mothers received intravenous cytarabine, alone or in combination with other agents, during the first trimester. The concern for fetal harm following intrathecal DepoCyt administration is low because systemic exposure to cytarabine is negligible. Cytarabine was teratogenic in mice (cleft palate, phocomelia, deformed appendages, skeletal abnormalities) when doses ≥2 mg/kg/day were administered IP during the period of organogenesis (about 0.2 times the recommended human dose on mg/m2 basis), and in rats (deformed appendages) when 20 mg/kg was administered as a single IP dose on day 12 of gestation (about 4 times the recommended human dose on mg/m2 basis). Single IP doses of 50 mg/kg in rats (about 10 times the recommended human dose on mg/m2 basis) on day 14 of gestation also cause reduced prenatal and postnatal brain size and permanent impairment of learning ability. Cytarabine was embryotoxic in mice when administered during the period of organogenesis. Embryotoxicity was characterized by decreased fetal weight at 0.5 mg/kg/day (about 0.05 times the recommended human dose on mg/m2 basis), and increased early and late resorptions and decreased live litter sizes at 8 mg/kg/day (approximately equal to the recommended human dose on mg/m2 basis). There are no adequate and well-controlled studies in pregnant women. If this drug is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential harm to the fetus. Despite the low apparent risk for fetal harm, women of childbearing potential should be advised to avoid becoming pregnant.
PRECAUTIONS
General Precautions
DepoCyt® (cytarabine liposome injection) has the potential of producing serious toxicity (see boxed WARNING). All patients receiving DepoCyt should be treated concurrently with dexamethasone to mitigate the symptoms of chemical arachnoiditis (see DOSAGE AND ADMINISTRATION). Toxic effects may be related to a single dose or to cumulative administration. Because toxic effects can occur at any time during therapy (although they are most likely to occur within 5 days of drug administration), patients receiving intrathecal therapy with DepoCyt should be monitored continuously for the development of neurotoxicity. If patients develop neurotoxicity, subsequent doses of DepoCyt should be reduced, and DepoCyt should be discontinued if toxicity persists.
Some patients with neoplastic meningitis receiving treatment with DepoCyt may require concurrent radiation or systemic therapy with other chemotherapeutic agents; this may increase the rate of adverse events.
Anaphylactic reactions following intravenous administration of free cytarabine have been reported.
Although significant systemic exposure to free cytarabine following intrathecal treatment is not expected, some effect on bone marrow function cannot be excluded. Systemic toxicity due to intravenous administration of cytarabine consists primarily of bone marrow suppression with leukopenia, thrombocytopenia, and anemia. Accordingly, careful monitoring of the hematopoietic system is advised.
Transient elevations in CSF protein and white blood cells have been observed in patients following DepoCyt administration and have also been noted after intrathecal treatment with methotrexate or cytarabine.
Information for the Patient
Patients should be informed about the expected adverse events of headache, nausea, vomiting, and fever, and about the early signs and symptoms of neurotoxicity. The importance of concurrent dexamethasone administration should be emphasized at the initiation of each cycle of DepoCyt treatment. Patients should be instructed to seek medical attention if signs or symptoms of neurotoxicity develop, or if oral dexamethasone is not well tolerated (see DOSAGE AND ADMINISTRATION).
Drug Interactions
No formal drug interaction studies of DepoCyt and other drugs were conducted. Concomitant administration of DepoCyt with other antineoplastic agents administered by the intrathecal route has not been studied. With intrathecal cytarabine and other cytotoxic agents administered intrathecally, enhanced neurotoxicity has been associated with coadministration of drugs.
Laboratory Test Interactions
Since DepoCyt particles are similar in size and appearance to white blood cells, care must be taken in interpreting CSF examinations following DepoCyt administration.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity, mutagenicity or impairment of fertility studies have been conducted with DepoCyt. The active ingredient of DepoCyt, cytarabine, was mutagenic in in vitro tests and was clastogenic in vitro (chromosome aberrations and SCE in human leukocytes) and in vivo (chromosome aberrations and SCE assay in rodent bone marrow, mouse micronucleus assay). Cytarabine caused the transformation of hamster embryo cells and rat H43 cells in vitro. Cytarabine was clastogenic to meiotic cells; a dose-dependent increase in sperm-head abnormalities and chromosomal aberrations occurred in mice given IP cytarabine. Impairment of Fertility: No studies assessing the impact of cytarabine on fertility are available in the literature. Because the systemic exposure to free cytarabine following intrathecal treatment with DepoCyt was negligible, the risk of impaired fertility after intrathecal DepoCyt is likely to be low.
Pregnancy
Pregnancy Category D (see WARNINGS).
Nursing Mothers
It is not known whether cytarabine is excreted in human milk following intrathecal DepoCyt administration. The systemic exposure to free cytarabine following intrathecal treatment with DepoCyt was negligible. Despite the low apparent risk, because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants, the use of DepoCyt is not recommended in nursing women.
Pediatric Use
The safety and efficacy of DepoCyt in pediatric patients has not been established.
ADVERSE REACTIONS
The toxicity database consists of the observations made during Phase 1-4 studies. The most common adverse reactions in all patients and in patients with lymphoma are shown in Table 2 below.
Arachnoiditis is an expected and well-documented side effect of both neoplastic meningitis and of intrathecal chemotherapy. The incidence of severe and life-threatening arachnoiditis in patients receiving DepoCyt was 19% (48/257) in all patients and 30% (10/33) in patients with lymphomatous meningitis. The incidence of symptoms possibly reflecting meningeal irritation are shown in Table 3.
In the early dose-finding study, chemical arachnoiditis was observed in 100% of cycles without dexamethasone prophylaxis. When concurrent dexamethasone was administered, chemical arachnoiditis was observed in 33% of cycles. Patients receiving DepoCyt should be treated concurrently with dexamethasone to mitigate the symptoms of chemical arachnoiditis (see DOSAGE AND ADMINISTRATION).
| Lymphoma | |||
| System Organ Class / Preferred Term | All DepoCyt (N=257) | DepoCyt (N=33) | Ara-C (N=28) |
| Nervous System Disorders | |||
| Headache NOS | 144 (56%) | 17 (52%) | 9 (32%) |
| Arachnoiditis | 108 (42%) | 14 (42%) | 10 (36%) |
| Confusion | 86 (33%) | 12 (36%) | 3 (11%) |
| Gait abnormal NOS | 60 (23%) | 7 (21%) | 8 (29%) |
| Convulsions NOS | 52 (20%) | 7 (21%) | 1 (4%) |
| Dizziness NOS | 47 (18%) | 7 (21%) | 6 (21%) |
| Memory impairment | 36 (14%) | 4 (12%) | 1 (4%) |
| Hypoaesthesia | 26 (10%) | 4 (12%) | 3 (11%) |
| Tremor | 22 (9%) | 5 (15%) | 5 (18%) |
| Peripheral neuropathy NOS | 9 (4%) | 4 (12%) | 1 (4%) |
| Syncope | 8 (3%) | 0 (0%) | 3 (11%) |
| Neuropathy NOS | 7 (3%) | 3 (9%) | 3 (11%) |
| Peripheral sensory neuropathy | 7 (3%) | 2 (6%) | 3 (11%) |
| Reflexes abnormal | 7 (3%) | 0 (0%) | 3 (11%) |
| General Disorders and Administration Site Conditions | |||
| Weakness | 103 (40%) | 13 (39%) | 15 (54%) |
| Pyrexia | 81 (32%) | 15 (45%) | 12 (43%) |
| Fatigue | 64 (25%) | 9 (27%) | 13 (46%) |
| Lethargy | 41 (16%) | 4 (12%) | 4 (14%) |
| Death NOS | 35 (14%) | 9 (27%) | 5 (18%) |
| Pain NOS | 35 (14%) | 3 (9%) | 5 (18%) |
| Oedema peripheral | 27 (11%) | 6 (18%) | 7 (25%) |
| Fall | 12 (5%) | 0 (0%) | 3 (11%) |
| Mucosal inflammation Nos | 8 (3%) | 4 (12%) | 2 (7%) |
| Oedema NOS | 6 (2%) | 1 (3%) | 6 (21%) |
| Gastrointestinal Disorders | |||
| Nausea | 117 (46%) | 11 (33%) | 15 (54%) |
| Vomiting NOS | 112 (44%) | 11 (33%) | 9 (32%) |
| Constipation | 64 (25%) | 8 (24%) | 7 (25%) |
| Diarrhoea NOS | 31 (12%) | 9 (27%) | 9 (32%) |
| Abdominal pain NOS | 22 (9%) | 5 (15%) | 4 (14%) |
| Dysphagia | 20 (8%) | 3 (9%) | 3 (11%) |
| Haemorrhoids | 8 (3%) | 0 (0%) | 3 (11%) |
| Musculoskeletal and Connective Tissue Disorders | |||
| Back pain | 61 (24%) | 7 (21%) | 5 (18%) |
| Pain in limb | 39 (15%) | 4 (12%) | 8 (29%) |
| Neck pain | 36 (14%) | 5 (15%) | 3 (11%) |
| Arthralgia | 29 (11%) | 3 (9%) | 4 (14%) |
| Neck stiffness | 28 (11%) | 2 (6%) | 4 (14%) |
| Muscle weakness NOS | 25 (10%) | 5 (15%) | 2 (7%) |
| Psychiatric Disorders | |||
| Insomnia | 35 (14%) | 6 (18%) | 7 (25%) |
| Agitation | 26 (10%) | 5 (15%) | 2 (7%) |
| Depression | 21 (8%) | 6 (18%) | 4 (14%) |
| Anxiety | 17 (7%) | 1 (3%) | 3 (11%) |
| Infections and Infestations | |||
| Urinary tract infection NOS | 35 (14%) | 6 (18%) | 5 (18%) |
| Pneumonia NOS | 16 (6%) | 2 (6%) | 3 (11%) |
| Metabolism and Nutrition Disorders | |||
| Dehydration | 33 (13%) | 6 (18%) | 3 (11%) |
| Appetite decreased NOS | 29 (11%) | 4 (12%) | 3 (11%) |
| Hyponatraemia | 18 (7%) | 4 (12%) | 1 (4%) |
| Hypokalaemia | 17 (7%) | 5 (15%) | 2 (7%) |
| Hyperglycaemia | 15 (6%) | 4 (12%) | 2 (7%) |
| Anorexia | 14 (5%) | 1 (3%) | 5 (18%) |
| Investigations | |||
| Platelet count decreased | 8 (3%) | 0 (0%) | 3 (11%) |
| Renal and Urinary Disorders | |||
| Incontinence NOS | 19 (7%) | 3 (9%) | 5 (18%) |
| Urinary retention | 14 (5%) | 0 (0%) | 3 (11%) |
| Respiratory, Thoracic and Mediastinal Disorders | |||
| Dyspnoea NOS | 25 (10%) | 4 (12%) | 6 (21%) |
| Cough | 17 (7%) | 3 (9%) | 6 (21%) |
| Eye Disorders | |||
| Vision blurred | 29 (11%) | 4 (12%) | 4 (14%) |
| Blood and Lymphatic Disorders | |||
| Anaemia NOS | 31 (12%) | 6 (18%) | 5 (18%) |
| Thrombocytopenia | 27 (11%) | 8 (24%) | 9 (32%) |
| Neutropenia | 26 (10%) | 12 (36%) | 7 (25%) |
| Skin and Subcutaneous Tissue Disorders | |||
| Contusion | 6 (2%) | 1 (3%) | 3 (11%) |
| Pruritus NOS | 6 (2%) | 0 (0%) | 4 (14%) |
| Sweating increased | 6 (2%) | 1 (3%) | 3 (11%) |
| Vascular Disorders | |||
| Hypotension NOS | 21 (8%) | 6 (18%) | 2 (7%) |
| Hypertension NOS | 15 (6%) | 5 (15%) | 1 (4%) |
| Ear and Labyrinth Disorders | |||
| Hypoacusis | 15 (6%) | 6 (18%) | 3 (11%) |
| Cardiac Disorders | |||
| Tachycardia NOS | 22 (9%) | 0 (0%) | 5 (18%) |
| Neoplasms Benign, Malignant and Unspecified (Incl Cysts and Polyps) | |||
| Diffuse Large B-Cell Lymphoma NOS | 1 (0%) | 1 (3%) | 3 (11%) |
|
* Hydrocephalus acquired, CSF pleocytosis and meningism occurred in ≤ 10% of all studied adult patients receiving DepoCyt or an active comparator |
|||||
| System Organ Class / Preferred Term | DepoCyt (N=257) | MTX (N=78) | Ara-C (N=28) |
||
| Nervous System Disorders | |||||
| Headache NOS | 145 (56%) | 33 (42%) | 9 (32%) | ||
| Arachnoiditis | 108 (42%) | 15 (19%) | 10 (36%) | ||
| Convulsions NOS | 56 (22%) | 11 (14%) | 1 (4%) | ||
| Gastrointestinal Disorders | |||||
| Nausea | 117 (46%) | 24 (31%) | 15 (54%) | ||
| Vomiting NOS | 112 (44%) | 22 (28%) | 9 (32%) | ||
| Musculoskeltal and Connective Tissue Disorders | |||||
| Back pain | 61 (24%) | 15 (19%) | 5 (18%) | ||
| Neck pain | 36 (14%) | 6 (8%) | 3 (11%) | ||
| Neck stiffness | 28 (11%) | 1 (1%) | 4 (14%) | ||
| General Disorders and Administration Site Conditions | |||||
| Pyrexia | 81 (32%) | 15 (19%) | 12 (43%) | ||
OVERDOSAGE
No overdosages with DepoCyt® (cytarabine liposome injection) have been reported. An overdose with DepoCyt may be associated with severe chemical arachnoiditis including encephalopathy.
In an early uncontrolled study without dexamethasone prophylaxis, single doses up to 125 mg were administered. One patient at the 125 mg dose level died of encephalopathy 36 hours after receiving an intraventricular dose of DepoCyt (see WARNINGS). This patient, however, was also receiving concomitant whole brain irradiation and had previously received intraventricular methotrexate.
There is no antidote for overdose of intrathecal DepoCyt or unencapsulated cytarabine released from DepoCyt. Exchange of CSF with isotonic saline has been carried out in a case of intrathecal overdose of free cytarabine, and such a procedure may be considered in the case of DepoCyt overdose. Management of overdose should be directed at maintaining vital functions.
DOSAGE AND ADMINISTRATION
Preparation of DepoCyt® (cytarabine liposome injection)
DepoCyt is a cytotoxic anticancer drug and, as with other potentially toxic compounds, caution should be used in handling DepoCyt. The use of gloves is recommended. If DepoCyt suspension contacts the skin, wash immediately with soap and water. If it contacts mucous membranes, flush thoroughly with water (see HANDLING AND DISPOSAL). DepoCyt particles are more dense than the diluent and have a tendency to settle with time. Vials of DepoCyt should be allowed to warm to room temperature and gently agitated or inverted to re-suspend the particles immediately prior to withdrawal from the vial. Avoid aggressive agitation. No further reconstitution or dilution is required.
DepoCyt Administration
DepoCyt should be withdrawn from the vial immediately before administration. DepoCyt is a single-use vial and does not contain any preservative; DepoCyt should be used within 4 hours of withdrawal from the vial. Unused portions of each vial should be discarded properly (see HANDLING AND DISPOSAL). Do not save any unused portions for later administration. Do not mix DepoCyt with any other medications.
In-line filters must not be used when administering DepoCyt. DepoCyt is administered directly into the CSF via an intraventricular reservoir or by direct injection into the lumbar sac. DepoCyt should be injected slowly over a period of 1-5 minutes. Following drug administration by lumbar puncture, the patient should be instructed to lie flat for 1 hour. Patients should be observed by the physician for immediate toxic reactions.
Patients should be started on dexamethasone 4 mg bid either PO or IV for 5 days beginning on the day of DepoCyt injection.
DepoCyt must only be administered by the intrathecal route.
Further dilution of DepoCyt is not recommended.
Dosing Regimen
For the treatment of lymphomatous meningitis, DepoCyt 50 mg (one vial of DepoCyt) is recommended to be given according to the following schedule:
Induction therapy: DepoCyt, 50 mg, administered intrathecally (intraventricular or lumbar puncture) every 14 days for 2 doses (weeks 1 and 3).
Consolidation therapy: DepoCyt, 50 mg, administered intrathecally (intraventricular or lumbar puncture) every 14 days for 3 doses (weeks 5, 7 and 9) followed by 1 additional dose at week 13.
Maintenance: DepoCyt, 50 mg, administered intrathecally (intraventricular or lumbar puncture) every 28 days for 4 doses (weeks 17, 21, 25 and 29).
If drug related neurotoxicity develops, the dose should be reduced to 25 mg. If it persists, treatment with DepoCyt should be discontinued.
HANDLING AND DISPOSAL
Procedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published.1-5 There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
HOW SUPPLIED
DepoCyt® (cytarabine liposome injection) is supplied as a sterile, white to off-white suspension in 5 mL glass, single use vials.
Refrigerate at 2° to 8°C (36° to 46°F). Protect from freezing and avoid aggressive agitation.
Available as individual carton containing one ready to use vial. NDC 57665-331-01.
Do not use beyond expiration date printed on the label.
REFERENCES:
- NIOSH Alert: Preventing occupational exposures to antineoplastic and other hazardous drugs in healthcare settings. 2004. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2004-165.
- OSHA Technical Manual, TED 1-0.15A, Section VI: Chapter 2. Controlling Occupational Exposure to Hazardous Drugs. OSHA, 1999. http://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2.html
- NIH [2002]. 1999 recommendations for the safe handling of cytotoxic drugs. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, NIH Publication No. 92-2621.
- American Society of Health-System Pharmacists. (2006) ASHP Guidelines on Handling Hazardous Drugs.
- Polovich, M., White, J. M., & Kelleher, L.O. (eds.) 2005. Chemotherapy and biotherapy guidelines and recommendations for practice (2nd. ed.) Pittsburgh, PA: Oncology Nursing Society.
Rx only
For additional information, contact Enzon Medical Information at: 866-792-5172
Manufactured by:
Pacira Pharmaceuticals Inc
San Diego, CA 92121
Distributed by:
ENZON Pharmaceuticals, Inc.
Bridgewater, NJ 08807
U.S. Patent Nos. 5,807,572; 5,723,147
April 2007 ©2000-2007 Pacira Pharmaceuticals Inc.
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 5mL VIAL Label

PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - CARTON, 5mL VIAL

| DEPOCYT
cytarabine injection, lipid complex |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA021041 | 04/01/1999 | |
| Labeler - Enzon Pharmaceutical, Inc. (101686731) |
| Registrant - Pacira Pharmaceuticals, Inc. (783298615) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Pacira Pharmaceuticals, Inc. | 783298615 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Catalent | 796436181 | LABEL | |