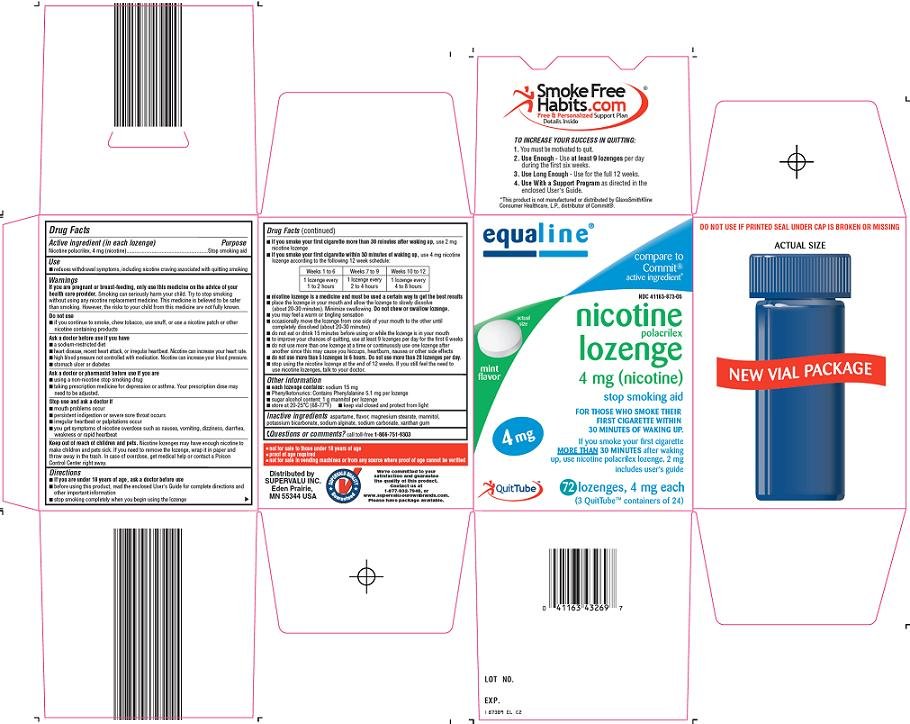
EQUALINE NICOTINE
-
nicotine lozenge
Supervalu Inc
----------
SuperValu Nicotine Polacrilex Lozenge 4 mg (nicotine) Drug FactsActive ingredient (in each lozenge)
Nicotine polacrilex, 4 mg (nicotine)
Purpose
Stop smoking aid
Uses
- reduces withdrawal symptoms, including nicotine craving associated with quitting smoking
Warnings
If you are pregnant or breast-feeding,
only use this medicine on the advice of your health care provider. Smoking can seriously harm your child. Try to stop smoking without using any nicotine replacement medicine. This medicine is believed to be safer than smoking. However, the risks to your child from this medicine are not fully known.
Do not use
- if you continue to smoke, chew tobacco, use snuff, or use a nicotine patch or other nicotine containing products
Ask a doctor before use if you have
- a sodium-restricted diet
- heart disease, recent heart attack, or irregular heartbeat. Nicotine can increase your heart rate.
- high blood pressure not controlled with medication. Nicotine can increase your blood pressure.
- stomach ulcer or diabetes
Ask a doctor or pharmacist before use if you are
- using a non-nicotine stop smoking drug
- taking prescription medicine for depression or asthma. Your prescription dose may need to be adjusted.
Stop use and ask a doctor if
- mouth problems occur
- persistent indigestion or severe sore throat occurs
- irregular heartbeat or palpitations occur
- you get symptoms of nicotine overdose such as nausea, vomiting, dizziness, diarrhea, weakness or rapid heartbeat
Keep out of reach of children and pets.
Nicotine lozenges may have enough nicotine to make children and pets sick. If you need to remove the lozenge, wrap it in paper and throw away in the trash. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- if you are under 18 years of age, ask a doctor before use
- before using this product, read the enclosed User’s Guide for complete directions and other important information
- stop smoking completely when you begin using the lozenge
- if you smoke your first cigarette more than 30 minutes after waking up, use 2 mg nicotine lozenge
- if you smoke your first cigarette within 30 minutes of waking up, use 4 mg nicotine lozenge according to the following 12 week schedule:
| Weeks 1 to 6 | Weeks 7 to 9 | Weeks 10 to 12 |
|
1 lozenge every 1 to 2 hours |
1 lozenge every 2 to 4 hours |
1 lozenge every 4 to 8 hours |
- nicotine lozenge is a medicine and must be used a certain way to get the best results
- place the lozenge in your mouth and allow the lozenge to slowly dissolve (about 20-30 minutes). Minimize swallowing. Do not chew or swallow lozenge.
- you may feel a warm or tingling sensation
- occasionally move the lozenge from one side of your mouth to the other until completely dissolved (about 20-30 minutes)
- do not eat or drink 15 minutes before using or while the lozenge is in your mouth
- to improve your chances of quitting, use at least 9 lozenges per day for the first 6 weeks
- do not use more than one lozenge at a time or continuously use one lozenge after another since this may cause you hiccups, heartburn, nausea or other side effects
- do not use more than 5 lozenges in 6 hours. Do not use more than 20 lozenges per day.
- stop using the nicotine lozenge at the end of 12 weeks. If you still feel the need to use nicotine lozenges, talk to your doctor.
Other information
- each lozenge contains: sodium 15 mg
- Phenylketonurics: Contains Phenylalanine 5.1 mg per lozenge
- sugar alcohol content: 1 g mannitol per lozenge
- store at 20-25°C (68-77°F)
- keep vial closed and {VIAL PACKAGING CONFIGURATION ONLY} protect from light
Inactive ingredients
aspartame, flavor, magnesium stearate, mannitol, potassium bicarbonate, sodium alginate, sodium carbonate, xanthan gum
Questions or comments?
call toll-free 1-866-751-9303
Principal Display Panel
Compare to Commit® active ingredient
Actual Size
Mint Flavor
Nicotine Polacrilex Lozenge 4 mg (nicotine)
Stop Smoking Aid
For those who smoke their first cigarette within 30 minutes of waking up.
If you smoke your first cigarette more than 30 minutes after waking up, use Nicotine Polacrilex Lozenge, 2 mg
Includes User’s Guide
4 mg
QuitTube {VIAL PACKAGING CONFIGURATION ONLY}
4 mg each
(# QuitTube™ containers of #) {VIAL PACKAGING CONFIGURATION ONLY}
Nicotine Polacrilex Lozenge 4 mg (nicotine) Carton
| EQUALINE NICOTINE
nicotine polacrilex lozenge |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA077007 | 03/28/2006 | |
| Labeler - Supervalu Inc (006961411) |