hypaque sodium (Diatrizoate Sodium) powder, for solution
[Amersham Health Inc.]
For Examination of the Gastrointestinal Tract
| NOT FOR INTRATHECAL USE |
RX ONLY
DESCRIPTION
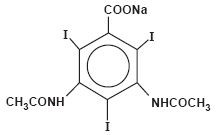
HYPAQUE Sodium (Diatrizoate Sodium, USP) is sodium 3,5-diacetamido-2, 4, 6-triiodobenzoate (C11H8I3N2NaO4) and contains 59.87 percent iodine. It is available as a powder. The powder (for preparing radiopaque solutions) provides about 600 mg organically bound iodine per gram of powder and contains caramel as a coloring agent. The structural formula is as follows:
Inactive Ingredients: Caramel, Polysorbate 80.
CLINICAL PHARMACOLOGY
When administered orally or given as an enema, the medium produces excellent opacification and delineation of the upper and lower gastrointestinal tract; however, because of dilution, contrast in the small bowel may be unsatisfactory. HYPAQUE solutions are particularly valuable when a more viscous agent, such as barium sulfate which is not water soluble, is unsuitable or potentially harmful.
From 0.04 to 1.2 percent of the medium may be absorbed from the gastrointestinal tract after oral administration. In some patients, particularly in infants and patients with engorgement of the intestinal mucosa, sufficient absorption to cause some visualization of the urinary tract may occur occasionally. HYPAQUE is also absorbed across the peritoneum and pleura.
INDICATIONS AND USAGE
This medium is indicated for radiographic examination of the gastrointestinal tract following oral or rectal administration.
WARNINGS
SEVERE ADVERSE EVENTS-INADVERTENT INTRATHECAL ADMINISTRATION
Severe adverse reactions have been reported due to the inadvertent intrathecal administration of iodinated contrast media that are not indicated for intrathecal use. These serious adverse reactions include: death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema. Special attention must be given to insure that this drug product is not administered intrathecally.
Serious or fatal reactions have been associated with the parenteral administration of radiopaque media. It is important that a course of action be carefully planned in advance for the treatment of possible serious reactions with oral use of HYPAQUE solutions. (See Precautions.)
Usage in Pregnancy
The safety of orally administered HYPAQUE solutions during pregnancy has not been established. Therefore, before administration of the drug to women of childbearing potential, the benefit to the patient should be carefully weighed against the possible risk to the fetus. In addition, most authorities consider elective contrast radiography of the abdomen contraindicated during pregnancy.
PRECAUTIONS
A 10 percent solution of the medium in water is isotonic. Hence, the solutions generally employed clinically (ie, from 15 to 40 percent) are hypertonic. Highly hypertonic solutions may draw excessive amounts of fluid into the intestine and may lead to hypovolemia. In very young or debilitated children, and in elderly cachectic persons, the loss of plasma fluid may be sufficient to cause a shock-like state which, if untreated, could be dangerous to the patient. Wherever possible, the use of the lower concentrations is advised for infants and young children (under 10 kg) and for dehydrated or debilitated patients. The higher concentrations should be used in such patients only when necessary and with great caution.
Therefore, it is advisable to correct any electrolyte disturbances before using solutions that are extremely hypertonic and to promptly correct hypovolemic disturbance caused by the medium.
Bronchial entry of the medium causes a copious osmotic effusion. Therefore, pulmonary entry by aspiration or by use in patients with esophagotracheal fistula should be avoided.
Caution is also advised in patients with severe renal or hepatic disease.
In patients with known hypersensitivity to diatrizoic acid compounds, the benefit to risk ratio should be considered.
ADVERSE REACTIONS
There have been few reported cases of adverse reactions with orally administered solutions of water-soluble contrast agents. Because of their osmotic effect, such solutions tend to exert cathartic action, but this is generally considered an advantage. Nausea, vomiting, or slight diarrhea may occasionally occur, particularly when the medium is used in a high concentration or large volume. Urticaria has been observed in a few patients; presumably the condition was caused by allergy to the contrast medium and it was readily alleviated by antihistamine therapy.
Because small amounts of the medium may be absorbed, the possibility of systemic reactions should be considered, particularly in cases of perforation.
DOSAGE AND ADMINISTRATION
Adults
Oral, from 90 mL to 180 mL of a 25 to 40 percent solution.
Enema, from 500 mL to 1000 mL of a 15 to 25 percent solution.
Infants and Children
Oral, from 30 mL to 75 mL of a 20 to 40 percent solution.
Enema, from 100 mL to 500 mL of a 10 to 15 percent solution, depending on weight of patient.
Warning
The powder is not to be used for the preparation of solution for parenteral injection.
| Solution (%) approx. | Measuring spoons* of powder (per 100 mL diluent †) |
|---|---|
|
|
| 10 | 1 |
| 15 | 1 1/2 |
| 20 | 2 |
| 25 | 2 1/2 |
| 40 | 4 |
HOW SUPPLIED
HYPAQUE Oral Powder contains 59.87 percent iodine or about 600 mg iodine per g.
HYPAQUE Oral Powder—Cans of 250 g with measuring spoon (approximately 10 g capacity), NDC 0407-0769-01.
Store in the dark at 20°-25°C (68°-77°F). [See USP controlled room temperature.]
Distributed by Amersham Health Inc.
Princeton, NJ 08540
Printed in USA
August 2004
HNC-6F
| Hypaque Sodium (Diatrizoate Sodium) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Revised: 02/2006