fortovase (Saquinavir) capsule, liquid filled
[Roche Laboratories Inc.]
Product identification in this document includes: INVIRASE® in reference to saquinavir mesylate; FORTOVASE in reference to saquinavir, and saquinavir in reference to the active base.
DESCRIPTION
FORTOVASE brand of saquinavir is an inhibitor of the human immunodeficiency virus (HIV) protease. FORTOVASE is available as beige, opaque, soft gelatin capsules for oral administration in a 200-mg strength (as saquinavir free base). Each capsule also contains the inactive ingredients medium chain mono- and diglycerides, povidone and dl-alpha tocopherol. Each capsule shell contains gelatin and glycerol 85% with the following colorants: red iron oxide, yellow iron oxide, and titanium dioxide. The chemical name for saquinavir is N-tert-butyl-decahydro-2-[2(R)-hydroxy-4-phenyl-3(S)-[[N-(2-quinolylcarbonyl)-L-asparaginyl]amino]butyl]-(4aS,8aS)-isoquinoline-3(S)-carboxamide which has a molecular formula C38H50N6O5 and a molecular weight of 670.86. Saquinavir has the following structural formula:
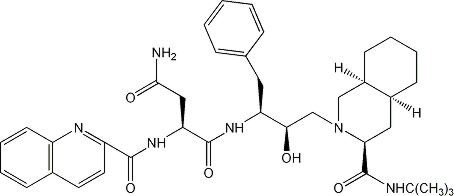
Saquinavir is a white to off-white powder and is insoluble in aqueous medium at 25°C.
MICROBIOLOGY
Mechanism of Action
Saquinavir is an inhibitor of HIV protease. HIV protease is an enzyme required for the proteolytic cleavage of viral polyprotein precursors into individual functional proteins found in infectious HIV. Saquinavir is a peptide-like substrate analogue that binds to the protease active site and inhibits the activity of the enzyme. Saquinavir inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature noninfectious virus particles.
Antiviral Activity
In vitro antiviral activity of saquinavir was assessed in lymphoblastoid and monocytic cell lines and in peripheral blood lymphocytes. Saquinavir inhibited HIV activity in both acutely and chronically infected cells. IC50 and IC90 values (50% and 90% inhibitory concentrations) were in the range of 1 to 30 nM and 5 to 80 nM, respectively. In the presence of 40% human serum, the mean IC50 of saquinavir against laboratory strain HIV-1 RF in MT4 cells was 37.7 ± 5 nM, representing a 4-fold increase in IC50 value. In cell culture, saquinavir demonstrated additive to synergistic effects against HIV-1 in combination with reverse transcriptase inhibitors (didanosine, lamivudine, nevirapine, stavudine, zalcitabine and zidovudine) without enhanced cytotoxicity. Saquinavir in combination with the protease inhibitors amprenavir, atazanavir, or lopinavir resulted in synergistic antiviral activity.
Drug Resistance
HIV-1 mutants with reduced susceptibility to saquinavir have been selected during in vitro passage. Genotypic analyses of these isolates showed several substitutions in the HIV protease gene. Only the G48V and L90M substitutions were associated with reduced susceptibility to saquinavir, and conferred an increase in the IC50 value of 8- and 3-fold, respectively.
HIV-1 isolates with reduced susceptibility (≥4-fold increase in the IC50 value) to saquinavir emerged in some patients treated with INVIRASE. Genotypic analysis of these isolates identified resistance conferring primary mutations in the protease gene G48V and L90M, and secondary mutations L10I/R/V, I54V/L, A71V/T, G73S, V77I, V82A and I84V that contributed additional resistance to saquinavir. Forty-one isolates from 37 patients failing therapy with INVIRASE had a median decrease in susceptibility to saquinavir of 4.3 fold.
The degree of reduction in in vitro susceptibility to saquinavir of clinical isolates bearing substitutions G48V and L90M depends on the number of secondary mutations present. In general, higher levels of resistance are associated with greater number of mutations only in association with either or both of the primary mutations G48V and L90M. No data are currently available to address the development of resistance in patients receiving saquinavir/ritonavir.
Cross-resistance
Among protease inhibitors, variable cross-resistance has been observed. In one clinical study, 22 HIV-1 isolates with reduced susceptibility (>4-fold increase in the IC50 value) to saquinavir following therapy with INVIRASE were evaluated for cross-resistance to amprenavir, indinavir, nelfinavir and ritonavir. Six of the 22 isolates (27%) remained susceptible to all 4 protease inhibitors, 12 of the 22 isolates (55%) retained susceptibility to at least one of the PIs and 4 out of the 22 isolates (18%) displayed broad cross-resistance to all PIs.
Sixteen (73%) and 11 (50%) of the 22 isolates remained susceptible (<4-fold) to amprenavir and indinavir, respectively. Four of 16 (25%) and 9 of 21 (43%) with available data remained susceptible to nelfinavir and ritonavir, respectively.
After treatment failure with amprenavir, cross-resistance to saquinavir was evaluated. HIV-1 isolates from 22/22 patients failing treatment with amprenavir and containing one or more mutations M46L/I , I50V, I54L, V32I, I47V, and I84V were susceptible to saquinavir.
CLINICAL PHARMACOLOGY
Pharmacokinetics
The pharmacokinetic properties of saquinavir when administered as FORTOVASE have been evaluated in healthy volunteers (n=207) and HIV-infected patients (n=91) after single-oral doses (range: 300 mg to 1200 mg) and multiple-oral doses (range: 400 mg to 1200 mg tid). The disposition properties of saquinavir have been studied in healthy volunteers after intravenous doses of 6, 12, 36 or 72 mg (n=21).
HIV-infected patients administered FORTOVASE (1200 mg tid) had AUC and maximum plasma concentration (Cmax) values approximately twice those observed in healthy volunteers receiving the same treatment regimen. The mean AUC values at week 1 were 4159 (CV 88%) and 8839 (CV 82%) ng∙h/mL, and Cmax values were 1420 (CV 81%) and 2477 (CV 76%) ng/mL for healthy volunteers and HIV-infected patients, respectively.
Absorption and Bioavailability in Adults
The absolute bioavailability of saquinavir administered as FORTOVASE has not been assessed. However, following single 600-mg doses, the relative bioavailability of saquinavir as FORTOVASE compared to saquinavir administered as INVIRASE was estimated as 331% (95% CI: 207% to 530%). The absolute bioavailability of saquinavir administered as INVIRASE averaged 4% (CV 73%, range: 1% to 9%) in 8 healthy volunteers who received a single 600-mg dose (3 x 200 mg) of INVIRASE following a high-fat breakfast (48 g protein, 60 g carbohydrate, 57 g fat; 1006 kcal). In healthy volunteers receiving single doses of FORTOVASE (300 mg to 1200 mg) and in HIV-infected patients receiving multiple doses of FORTOVASE (400 mg to 1200 mg tid), a greater than dose-proportional increase in saquinavir plasma concentrations has been observed.
Comparison of pharmacokinetic parameters between single- and multiple-dose studies shows that following multiple dosing of FORTOVASE (1200 mg tid) in healthy male volunteers (n=18), the steady-state AUC was 80% (95% CI: 22% to 176%) higher than that observed after a single 1200-mg dose (n=30).
Saquinavir plasma concentrations remained stable over a 60-week period of continued treatment in patients in a phase III substudy.
When administered as the sole protease inhibitor, it has been shown that FORTOVASE 1200 mg tid provides an 8-fold increase in AUC compared with INVIRASE 600 mg tid.
FORTOVASE in combination with ritonavir at doses of 400/400 mg bid, or 1000/100 mg bid provide saquinavir systemic exposures over a 24-hour period similar to or greater than those achieved with FORTOVASE 1200 mg tid.
| Dosing Regimen | n | AUC0-τ
(ng∙h/mL) | AUC0–24h
(ng∙h/mL) | Cmin
(ng/mL) |
|---|---|---|---|---|
| τ is the dosing interval (ie, 8h if tid and 12h if bid). | ||||
| FORTOVASE 1200 mg tid (arithmetic mean) | 31 | 7249 | 21747 | 216 |
| INVIRASE 400 mg bid + ritonavir 400 mg bid (arithmetic mean± SD) | 7 | 16000±8000 | 32000 | 480±360 |
| INVIRASE 1000 mg bid + ritonavir 100 mg bid (geometric mean and 95% CI) | 24 | 14607 (10218-20882 | 29214 | 371 (245-561) |
| FORTOVASE 1000 mg bid + ritonavir 100 mg bid (geometric mean and 95% CI) | 24 | 19085 (13943-26124) | 38170 | 433 (301-622) |
Food Effect
The mean 12-hour AUC after a single 800-mg oral dose of saquinavir in healthy volunteers (n=12) was increased from 167 ng∙h/mL (CV 45%), under fasting conditions, to 1120 ng∙h/mL (CV 54%) when FORTOVASE was given following a high-fat breakfast (45 g protein, 76 g carbohydrate, 55 g fat; 961 kcal). The effect of food with INVIRASE has been shown to persist for up to 2 hours. The mean 12-hour AUC after a single 1200-mg oral dose of FORTOVASE in healthy volunteers (n=12) was increased from 952 ng∙h/mL, following a light meal (21 g protein, 50 g carbohydrate, 28 g fat; 524 kcal) to 1388 ng∙h/mL when FORTOVASE was given following a high-fat breakfast (45 g protein, 76 g carbohydrate, 55 g fat; 961 kcal).
Saquinavir exposure was similar when FORTOVASE plus ritonavir (1000-mg/100-mg bid) was administered following a high-fat (45 g fat) or moderate-fat (20 g fat) breakfast.
Distribution in Adults
The mean steady-state volume of distribution following intravenous administration of a 12-mg dose of saquinavir (n=8) was 700 L (CV 39%), suggesting saquinavir partitions into tissues. It has been shown that saquinavir, up to 30 µg/mL, is approximately 97% bound to plasma proteins.
Metabolism and Elimination in Adults
In vitro studies using human liver microsomes have shown that the metabolism of saquinavir is cytochrome P450 mediated with the specific isoenzyme, CYP3A4, responsible for more than 90% of the hepatic metabolism. Based on in vitro studies, saquinavir is rapidly metabolized to a range of mono- and di-hydroxylated inactive compounds. In a mass balance study using 600 mg 14C-saquinavir mesylate (n=8), 88% and 1% of the orally administered radioactivity was recovered in feces and urine, respectively, within 5 days of dosing. In an additional 4 subjects administered 10.5 mg 14C-saquinavir intravenously, 81% and 3% of the intravenously administered radioactivity was recovered in feces and urine, respectively, within 5 days of dosing. In mass balance studies, 13% of circulating radioactivity in plasma was attributed to unchanged drug after oral administration and the remainder attributed to saquinavir metabolites. Following intravenous administration, 66% of circulating radioactivity was attributed to unchanged drug and the remainder attributed to saquinavir metabolites, suggesting that saquinavir undergoes extensive first-pass metabolism.
Systemic clearance of saquinavir was rapid, 1.14 L/h/kg (CV 12%) after intravenous doses of 6, 36, and 72 mg. The mean residence time of saquinavir was 7 hours (n=8).
Special Populations
Hepatic or Renal Impairment
Saquinavir pharmacokinetics in patients with hepatic or renal impairment has not been investigated (see PRECAUTIONS). Only 1% of saquinavir is excreted in the urine, so the impact of renal impairment on saquinavir elimination should be minimal.
Gender, Race and Age
The effect of gender was investigated in healthy volunteers receiving single 1200-mg doses of FORTOVASE (n=12 females, 18 males). No effect of gender was apparent on the pharmacokinetics of saquinavir in this study.
The effect of race on the pharmacokinetics of saquinavir has not been investigated.
Pediatric Patients
The pharmacokinetics of saquinavir in pediatric patients differs significantly from that in adults. Children have a markedly higher apparent clearance than adults and administration of saquinavir alone will not give consistently therapeutic plasma levels. The pharmacokinetics of saquinavir when coadministered with ritonavir to pediatric patients is under investigation.
Geriatric Patients
The pharmacokinetics of saquinavir when administered as FORTOVASE have not been sufficiently investigated in patients >65 years of age.
Drug Interactions
(see PRECAUTIONS: Drug Interactions)
It is important to be aware that, when coadministered with ritonavir, the occurrence and magnitude of drug interactions may differ from those seen with FORTOVASE when administered as the sole protease inhibitor. When ritonavir is coadministered, prescribers should refer to the prescribing information for ritonavir regarding drug interactions associated with this drug.
Table 2 summarizes the effect of FORTOVASE on the geometric mean AUC and Cmax of coadministered drugs. Table 3 summarizes the effect of coadministered drugs on the geometric mean AUC and Cmax of saquinavir.
| Coadministered Drug | FORTOVASE or FORTOVASE/ritonavir Dose | N | % Change for Coadministered Drug | |
|---|---|---|---|---|
| AUC (95%CI) | Cmax (95%CI) | |||
| ↑ Denotes an average increase in exposure by the percentage indicated. | ||||
| ↓ Denotes an average decrease in exposure by the percentage indicated. | ||||
| ↔ Denotes no statistically significant change in exposure was observed. | ||||
| P Patient | ||||
| V Healthy Volunteers | ||||
| Φ No longer marketed in the US. | ||||
|
||||
| Clarithromycin 500 mg bid × 7 days Clarithromycin 14-OH clarithromycin metabolite | 1200 mg tid × 7days | 12V |
↑45% (17-81%) ↓24% (5-40%) |
↑39% (10-76%) ↓34% (14-50%) |
| Midazolam 7.5-mg oral single dose | 1200 mg tid × 5days | 6V | ↑514% | ↑235% |
| Nelfinavir 750-mg single dose | 1200 mg tid × 4days | 14P | ↑18%(5-33%) | ↔ |
| Rifabutin 300 mg once daily | 1200 mg tid | 14P | ↑44% | ↑45% |
| Ritonavir 400 mg bid × 14 days | 400 mg bid × 14 days | 8V | ↔ | ↔ |
| Sildenafil 100-mg single dose | 1200 mg tid × 8 days | 27V | ↑210%(150-300%) | ↑140%(80-230%) |
| TerfenadineΦ 60 mg bid × 11 days*
Terfenadine Terfenadine acid metabolite | 1200 mg tid × 4 days | 12V |
↑368% (257-514%) ↑120% (89-156%) |
↑253% (164-373%) ↑93% (59-133%) |
| Efavirenz 600 mg | 1200 mg tid | 13V | ↓12% | ↓13% |
| Ketoconazole 400 mg once daily | 1200 mg tid | 12V | ↔ | ↔ |
| Enfuvirtide 90 mg SC q12h (bid) for 7 days | 1000/100 mg bid | 12 P | ↔ | ↔ |
| Coadministered Drug | FORTOVASE Dose | N | % Change for Saquinavir | |
|---|---|---|---|---|
| AUC (95%CI) | Cmax (95%CI) | |||
| ↑ Denotes an average increase in exposure by the percentage indicated. | ||||
| ↓ Denotes an average decrease in exposure by the percentage indicated. | ||||
| P Patient | ||||
| V Healthy Volunteers | ||||
| Clarithromycin 500 mg bid × 7 days | 1200 mg tid × 7days | 12V | ↑177% (108-269%) | ↑187% (105-300%) |
| Efavirenz 600 mg | 1200 mg tid | 13V | ↓62% | ↓50% |
| Indinavir 800 mg q8h × 2 days | 1200-mg single dose | 6V | ↑364% (190-644%) | ↑299% (138-568%) |
| Ketoconazole 400 mg once daily | 1200 mg tid | 12V | ↑190% | ↑171% |
| Nelfinavir 750 mg × 4 days | 1200-mg single dose | 14P | ↑392% (271-553%) | ↑179% (105-280%) |
| Rifabutin 300 mg once daily | 1200 mg tid | 14P | ↓47% | ↓31% |
| Rifampin 600 mg once daily | 1200 mg tid × 14 days | 14V | ↓70% | ↓65% |
| Ritonavir 100 mg bid | 1000 mg bid* | 24P | ↑176% | ↑153% |
| Ritonavir 400 mg bid × 14 days† | 400 mg bid × 14 days* | 8V | ↑121% (7-359%) | ↑64%§ |
| Lopinavir/ritonavir 400/100 mg bid, 15 days |
800 mg bid, 10 days combo vs. 1200 mg tid, 5 days alone |
14V |
↑9.62-fold (8.05, 11.49)‡ |
↑6.34-fold (5.32, 7.55)‡ |
| 400/100 mg bid, 20 days | 1200 mg bid, 5 days combo vs. 1200 mg tid 5 days alone | 10V | ↑9.91-fold (8.28, 11.86)‡ | ↑6.44-fold (5.59, 7.41)‡ |
For information regarding clinical recommendations, see PRECAUTIONS: Drug Interactions, Table 6.
INDICATIONS AND USAGE
FORTOVASE is indicated for use in combination with other antiretroviral agents for the treatment of HIV infection. This indication is based on studies that showed increased saquinavir concentrations and improved antiviral activity for FORTOVASE 1200 mg tid compared to INVIRASE 600 mg tid.
In treatment-naive and treatment-experienced patients, the efficacy of FORTOVASE (with or without ritonavir coadministration) has not been compared against the efficacy of antiretroviral regimens currently considered standard of care.
Description of Clinical Studies
When used in combination with other antiretroviral agents, FORTOVASE and INVIRASE have been shown to decrease plasma HIV RNA levels and increase CD4 cell counts in an open-label randomized study (NV15355) in treatment-naive, HIV-infected patients. In addition, in a randomized, double-blind study (NV14256) in ZDV-experienced, HIV-infected patients, a combination regimen of FORTOVASE and HIVID was shown to be superior to either INVIRASE or HIVID monotherapy in decreasing the cumulative incidence of clinical disease progression to AIDS-defining events or death. It should be noted that HIV treatment regimens that were used in the initial clinical studies of INVIRASE are no longer considered standard of care.
FORTOVASE 1000 mg bid coadministered with ritonavir 100 mg bid was studied in a heterogeneous population of 148 HIV infected patients (MaxCmin 1 study). At baseline 42 were treatment naive and 106 were treatment experienced (of which 52 had an HIV RNA level <400 copies/mL at baseline). Results showed that 91/148 (61%) subjects achieved and/or sustained and HIV RNA level <400 copies/mL at the completion of 48 weeks.
Study NV15182 was an open-label safety study of FORTOVASE in combination with other antiretroviral agents in HIV-infected patients. The 48-week safety results from this study are displayed in the ADVERSE REACTIONS section.
CONTRAINDICATIONS
FORTOVASE is contraindicated in patients with clinically significant hypersensitivity to saquinavir or to any of the components contained in the capsule.
FORTOVASE should not be administered concurrently with terfenadine, cisapride, astemizole, pimozide, triazolam, midazolam, or ergot derivatives, because competition for CYP3A4 by saquinavir could result in inhibition of the metabolism of these drugs and create the potential for serious and/or life-threatening reactions, such as cardiac arrhythmias or prolonged sedation (see PRECAUTIONS: Drug Interactions).
FORTOVASE is contraindicated in patients with severe hepatic impairment (see PRECAUTIONS: Hepatic Effects).
FORTOVASE should not be administered concurrently with drugs listed in Table 4 (also see PRECAUTIONS: Drug Interactions, Table 5).
| Drug Class | Drugs Within Class That Are Contraindicated With FORTOVASE |
|---|---|
|
|
| Antiarrhythmics | Amiodarone, bepridil, flecainide, propafenone, quinidine |
| Antihistamines | Astemizole*, terfenadine* |
| Ergot Derivatives | Dihydroergotamine, ergonovine, ergotamine, methylergonovine |
| Antimycobacterial Agents | Rifampin |
| GI Motility Agent | Cisapride* |
| Neuroleptics | Pimozide |
| Sedative/Hypnotics | Triazolam, midazolam |
If FORTOVASE is coadministered with ritonavir, the ritonavir label should be reviewed for additional contraindicated drugs.
WARNINGS
ALERT: Find out about medicines that should not be taken with FORTOVASE. This statement is included on the product's bottle label.
Interaction with HMG-CoA Reductase Inhibitors
Concomitant use of FORTOVASE with lovastatin or simvastatin is not recommended. Caution should be exercised if HIV protease inhibitors, including FORTOVASE, are used concurrently with other HMG-CoA reductase inhibitors that are also metabolized by the CYP3A4 pathway (eg, atorvastatin). Since increased concentrations of statins can, in rare cases, cause severe adverse events such as myopathy including rhabdomyolysis, this risk may be increased when HIV protease inhibitors, including saquinavir, are used in combination with these drugs.
Interaction with St. John's Wort (hypericum perforatum)
Concomitant use of FORTOVASE and St. Johns wort (hypericum perforatum) or products containing St. John's wort is not recommended. Coadministration of protease inhibitors, including FORTOVASE, with St. John's wort is expected to substantially decrease protease inhibitor concentrations and may result in sub-optimal levels of FORTOVASE and lead to loss of virologic response and possible resistance to FORTOVASE or to the class of protease inhibitors.
Interaction with Garlic Capsules
Garlic capsules should not be used while taking saquinavir as the sole protease inhibitor due to the risk of decreased saquinavir plasma concentrations. No data are available for the coadministration of FORTOVASE/ritonavir or INVIRASE/ritonavir and garlic capsules.
Diabetes Mellitus and Hyperglycemia
New onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus and hyperglycemia have been reported during postmarketing surveillance in HIV-infected patients receiving protease-inhibitor therapy. Some patients required either initiation or dose adjustments of insulin or oral hypoglycemic agents for the treatment of these events. In some cases diabetic ketoacidosis has occurred. In those patients who discontinued protease-inhibitor therapy, hyperglycemia persisted in some cases. Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made and a causal relationship between protease-inhibitor therapy and these events has not been established.
PRECAUTIONS
General
FORTOVASE (saquinavir) soft gelatin capsules and INVIRASE (saquinavir mesylate) capsules are not bioequivalent and cannot be used interchangeably when used as the sole protease inhibitor. Only FORTOVASE should be used for the initiation of therapy that includes saquinavir as a sole protease inhibitor (see DOSAGE AND ADMINISTRATION) since FORTOVASE soft gelatin capsules provide greater bioavailability and efficacy than INVIRASE capsules.
If a serious or severe toxicity occurs during treatment with FORTOVASE, FORTOVASE should be interrupted until the etiology of the event is identified or the toxicity resolves. At that time, resumption of treatment with full-dose FORTOVASE may be considered. For antiretroviral agents used in combination with FORTOVASE, physicians should refer to the complete product information for these drugs for dose adjustment recommendations and for information regarding drug-associated adverse reactions.
Hepatic Effects
The use of FORTOVASE by patients with hepatic impairment has not been studied. In the absence of such studies, caution should be exercised, as increases in saquinavir levels and/or increases in liver enzymes may occur. In patients with underlying hepatitis B or C, cirrhosis, chronic alcoholism and/or other underlying liver abnormalities there have been reports of worsening liver disease.
Renal Effects
Renal clearance is only a minor elimination pathway; the principal route of metabolism and excretion for saquinavir is by the liver. Therefore, no dose adjustment is necessary for patients with renal impairment. However, patients with severe renal impairment have not been studied and caution should be exercised when prescribing saquinavir in this population.
Hemophilia
There have been reports of spontaneous bleeding in patients with hemophilia A and B treated with protease inhibitors. In some patients additional factor VIII was required. In the majority of reported cases treatment with protease inhibitors was continued or restarted. A causal relationship between protease-inhibitor therapy and these episodes has not been established.
Hyperlipidemia
Elevated cholesterol and/or triglyceride levels have been observed in some patients taking saquinavir in combination with ritonavir. Marked elevation in triglyceride levels is a risk factor for development of pancreatitis. Cholesterol and triglyceride levels should be monitored prior to initiating combination dosing regimen of FORTOVASE or INVIRASE with ritonavir, and at periodic intervals while on such therapy. In these patients, lipid disorders should be managed as clinically appropriate.
Fat Redistribution
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), facial wasting, peripheral wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving antiretroviral therapy. A causal relationship between protease inhibitor therapy and these events has not been established and the long-term consequences are currently unknown.
Resistance/Cross-resistance
Varying degrees of cross-resistance among protease inhibitors have been observed. Continued administration of FORTOVASE therapy following loss of viral suppression may increase the likelihood of cross-resistance to other protease inhibitors (see MICROBIOLOGY).
Information For Patients
A statement to patients and health care providers is included on the product's bottle label: ALERT: Find out about medicines that should NOT be taken with FORTOVASE. A Patient Package Insert (PPI) for FORTOVASE is available for patient information.
Patients should be informed that any change from INVIRASE to FORTOVASE or FORTOVASE to INVIRASE coadministered with ritonavir should be made only under the supervision of a physician.
FORTOVASE may interact with some drugs; therefore, patients should be advised to report to their doctor the use of any other prescription, nonprescription medication, or herbal products, particularly St. John's wort.
Patients should be informed that FORTOVASE is not a cure for HIV infection and that they may continue to acquire illnesses associated with advanced HIV infection, including opportunistic infections. Patients should be advised that FORTOVASE should be used only in combination with other active antiretroviral medications.
Patients should be informed that redistribution or accumulation of body fat may occur in patients receiving protease inhibitors and that the cause and long-term health effects of these conditions are not known at this time.
Patients should be told that the long-term effects of FORTOVASE are unknown at this time. They should be informed that FORTOVASE therapy has not been shown to reduce the risk of transmitting HIV to others through sexual contact or blood contamination.
Patients should be advised that FORTOVASE should be taken within 2 hours after a full meal. When FORTOVASE is coadministered with ritonavir a light meal is sufficient (see CLINICAL PHARMACOLOGY: Pharmacokinetics). Patients should be advised of the importance of taking their medication every day, as prescribed, to achieve maximum benefit. Patients should not alter the dose or discontinue therapy without consulting their physician. If a dose is missed, patients should take the next dose as soon as possible. However, the patient should not double the next dose.
Patients should be informed that refrigerated (36° to 46°F, 2° to 8°C) capsules of FORTOVASE remain stable until the expiration date printed on the label. Once brought to room temperature [at or below 77°F (25°C)], capsules should be used within 3 months.
Laboratory Tests
Clinical chemistry tests, viral load, and CD4 count should be performed prior to initiating FORTOVASE therapy and at appropriate intervals thereafter. Elevated nonfasting triglyceride levels have been observed in patients in saquinavir trials. Triglyceride levels should be periodically monitored during therapy. For comprehensive information concerning laboratory test alterations associated with use of other antiretroviral therapies, physicians should refer to the complete product information for these drugs.
Drug Interactions
Several drug interaction studies have been completed with both INVIRASE and FORTOVASE. Observations from drug interaction studies with FORTOVASE may not be predictive for INVIRASE. If ritonavir is coadministered, prescribers should also refer to the prescribing information for ritonavir regarding drug interactions associated with this agent.
The metabolism of saquinavir is mediated by cytochrome P450, with the specific isoenzyme CYP3A4 responsible for 90% of the hepatic metabolism. Additionally, saquinavir is a substrate for P-Glycoprotein (Pgp). Therefore, drugs that affect CYP3A4 and/or Pgp, may modify the pharmacokinetics of saquinavir. Similarly, saquinavir might also modify the pharmacokinetics of other drugs that are substrates for CYP3A4 or Pgp.
Drugs that are contraindicated specifically due to the expected magnitude of interaction and potential for serious adverse events are listed in Table 4 under CONTRAINDICATIONS. Additional drugs that are not recommended for coadministration with FORTOVASE are included in Table 5. These recommendations are based on either drug interaction studies or predicted interactions due to the expected magnitude of interaction and potential for serious events or loss of efficacy.
Drug interactions that have been established based on drug interaction studies are listed with the pharmacokinetic results in Table 2, which summarizes the effect of saquinavir, administered as FORTOVASE, on the geometric mean AUC and Cmax of coadministered drugs and Table 3, which summarizes the effect of coadministered drugs on the geometric mean AUC and Cmax of saquinavir. Clinical dose recommendations can be found in Table 6. The magnitude of interactions may be different when FORTOVASE is given with ritonavir.
| Drug Class: Drug Name | Clinical Comment |
|---|---|
|
|
| Antiarrhythmics: Amiodarone, bepridil, flecainide, propafenone, quinidine | CONTRAINDICATED due to potential for serious and/or life-threatening reactions. |
| Antihistamines: astemizole*, terfenadine* | CONTRAINDICATED due to potential for serious and/or life-threatening cardiac arrhythmias. |
| Ergot Derivatives: Dihydroergotamine, ergonovine, ergotamine, methylergonovine | CONTRAINDICATED due to potential for serious and life-threatening reactions such as acute ergot toxicity characterized by peripheral vasospasm and ischemia of the extremities and other tissues. |
| Antimycobacterial Agents: rifampin | WARNING coadministration with rifampin is not recommended because rifampin markedly decreases the concentration of saquinavir. The safety and efficacy of this combination have not been established. |
| Garlic Capsules | Garlic capsules should not be used while taking saquinavir (FORTOVASE) as the sole protease inhibitor due to the risk of decreased saquinavir plasma concentrations. No data are available for the coadministration of INVIRASE/ritonavir or FORTOVASE/ritonavir and garlic capsules. |
| GI Motility Agent: cisapride* | CONTRAINDICATED due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias. |
| Herbal Products: St. John's wort (hypericum perforatum) | WARNING coadministration may lead to loss of virologic response and possible resistance to FORTOVASE or to the class of protease inhibitors. |
| HMG-CoA Reductase Inhibitors: lovastatin, simvastatin | WARNING potential for serious reactions such as risk of myopathy including rhabdomyolysis. |
| Sedatives/Hypnotics: triazolam, midazolam | CONTRAINDICATED due to potential for serious and/or life-threatening reactions such as prolonged or increased sedation or respiratory depression. |
If FORTOVASE is coadministered with ritonavir, the ritonavir label should be reviewed for additional drugs that should not be coadministered.
| Concomitant Drug Class: Drug Name | Effect on Concentration of Saquinavir or Concomitant Drug | Clinical Comment |
|---|---|---|
| HIV-Antiviral Agents | ||
|
||
| Non-nucleoside reverse transcriptase inhibitor:
Delavirdine | FORTOVASE
↑ Saquinavir Effect on delavirdine is not well established FORTOVASE/ritonavir Interaction has not been evaluated | Appropriate doses of the combination with respect to safety and efficacy have not been established. |
| Non-nucleoside reverse transcriptase inhibitor:
Efavirenz*, nevirapine | FORTOVASE
↓ Saquinavir ↓Efavirenz FORTOVASE/ritonavir Interaction has not been evaluated | FORTOVASE should not be given as the sole protease inhibitor to patients taking efavirenz or nevirapine. Appropriate doses of the combination of efavirenz or nevirapine and FORTOVASE/ritonavir with respect to safety and efficacy have not been established. |
| HIV protease inhibitor:
Indinavir* | FORTOVASE
↑ Saquinavir Effect on indinavir is not well established FORTOVASE/ritonavir Interaction has not been evaluated | Appropriate doses of the combination with respect to safety and efficacy have not been established. |
| HIV protease inhibitor:
Nelfinavir* | FORTOVASE
↑ Saquinavir ↑ Nelfinavir FORTOVASE/ritonavir Interaction has not been evaluated | Saquinavir 1200 mg bid with nelfinavir 1250 mg bid results in adequate plasma drug concentrations for both protease inhibitors. |
| HIV protease inhibitor:
Ritonavir* | FORTOVASE
↑ Saquinavir ↔ Ritonavir | The recommended dose regimen when ritonavir is given to increase saquinavir concentrations is 1000 mg saquinavir plus ritonavir 100 mg twice daily. |
| HIV protease inhibitor:
Lopinavir/ritonavir (coformulated capsule)* | FORTOVASE
↑ Saquinavir Effect on lopinavir is not well established | FORTOVASE (SQV) 800 mg bid + KALETRA produces ↑ AUC, ↑ Cmax, and ↑ Cmin relative to FORTOVASE 1200 mg tid (see CLINICAL PHARMACOLOGY: Table 3) |
| HIV fusion inhibitor:
Enfuvirtide* | FORTOVASE
Interaction has not been evaluated FORTOVASE/ritonavir ↔ Enfuvirtide |
No clinically significant interaction was noted from a study in 12 HIV patients who received enfuvirtide concomitantly with FORTOVASE/ritonavir 1000/100 mg bid. No dose adjustments are required. |
| Other Agents | ||
| Antiarrhythmics:
Lidocaine (systemic) | ↑ Antiarrhythmics | Caution is warranted and therapeutic concentration monitoring, if available, is recommended for antiarrhythmics given with FORTOVASE or FORTOVASE/ritonavir |
| Anticoagulant:
Warfarin | Concentrations of warfarin may be affected. It is recommended that INR (international normalized ratio) be monitored. | |
| Anticonvulsants: Carbamazepine, phenobarbital, phenytoin | FORTOVASE
↓ Saquinavir Effect on carbamazepine, phenobarbital, and phenytoin is not well established FORTOVASE/ritonavir Interaction has not been evaluated | Use with caution, FORTOVASE may be less effective due to decreased saquinavir plasma concentrations in patients taking these agents concomitantly. |
| Anti-infective:
Clarithromycin* | FORTOVASE
↑ Saquinavir ↑ Clarithromycin FORTOVASE/ritonavir Interaction has not been evaluated | No dose adjustment is required when the two drugs are coadministered for a limited time at the doses studied (clarithromycin 500 mg bid and FORTOVASE 1200 mg tid for 7 days). For patients with renal impairment, the following dosage adjustments should be considered:
|
| Antifungal:
Ketoconazole*, itraconazole | FORTOVASE
↑ Saquinavir ↔ Ketoconazole FORTOVASE/ritonavir Interaction has not been evaluated | No dose adjustment is required when the two drugs are coadministered for a limited time at the doses studied (ketoconazole 400 mg qd and FORTOVASE 1200 mg tid). A similar increase in plasma concentrations of saquinavir could occur with itraconazole. |
| Antimycobacterial
Rifabutin* | ↓ Saquinavir ↑Rifabutin | FORTOVASE should not be given as the sole protease inhibitor to patients taking rifabutin. Appropriate doses of the combination of rifabutin and FORTOVASE/ritonavir with respect to safety and efficacy have not been established. |
| Antimycobacterial
Rifampin* | FORTOVASE
↓ Saquinavir FORTOVASE/ritonavir Interaction has not been evaluated | FORTOVASE should not be given as the sole protease inhibitor to patients taking rifampin. Appropriate doses of the combination of rifampin and FORTOVASE/ritonavir with respect to safety and efficacy have not been established. |
| Benzodiazepines:
Alprazolam, clorazepate, diazepam, flurazepam | ↑ Benzodiazepines | Clinical significance is unknown; however, a decrease in benzodiazepine dose may be needed. |
| Calcium channel blockers:
Diltiazem, felodipine, nifedipine, nicardipine, nimodipine, verapamil, amlodipine, nisoldipine, isradipine | ↑ Calcium channel blockers | Caution is warranted and clinical monitoring of patients is recommended. |
| Corticosteroid:
Dexamethasone | FORTOVASE
↓ Saquinavir FORTOVASE/ritonavirInteraction has not been evaluated | Use with caution, FORTOVASE may be less effective due to decreased saquinavir plasma concentrations in patients taking these agents concomitantly. |
| Histamine H2-receptor antagonist:
Ranitidine | FORTOVASE
↑ Saquinavir FORTOVASE/ritonavirInteraction has not been evaluated | The increase is not thought to be clinically relevant and no dose adjustment of FORTOVASE is recommended. Appropriate doses of the combination of ranitidine and FORTOVASE/ritonavir with respect to safety and efficacy have not been established. |
| HMG-CoA reductase inhibitors:
Simvastatin, lovastatin, atorvastatin | ↑ HMG-CoA reductase inhibitors | The combination of FORTOVASE with simvastatin and lovastatin should be avoided. Use lowest possible dose of atorvastatin and with careful monitoring or consider other HMG-CoA reductase inhibitors such as pravastatin, fluvastatin and rosuvastatin. |
| Immunosuppressants:
Cyclosporine, tacrolimus, rapamycin | ↑ Immunosuppressants | Therapeutic concentration monitoring is recommended for immunosuppressant agents when coadministered with FORTOVASE or FORTOVASE/ritonavir. |
| Narcotic analgesic:
Methadone | FORTOVASE/ritonavir
↓ Methadone | Dosage of methadone may need to be increased when coadministered with FORTOVASE/ritonavir. |
| Oral contraceptives:
Ethinyl estradiol | FORTOVASE/ritonavir
↓ Ethinyl estradiol | Alternative or additional contraceptive measures should be used when estrogen-based oral contraceptives and FORTOVASE/ritonavir are coadministered. |
| PDE5 inhibitor (phosphodiesterase type 5 inhibitors):
Sildenafil*, vardenafil, tadalafil | ↑ Sildenafil ↔ Saquinavir ↑ Vardenafil ↑ Tadalafil FORTOVASE/ritonavir Interaction has not been evaluated, but expect increased concentrations of PDE5 inhibitors. | Use sildenafil with caution at reduced doses of 25 mg every 48 hours with increased monitoring of adverse events when administered concomitantly with FORTOVASE or FORTOVASE/ritonavir. Use vardenafil with caution at reduced doses of no more than 2.5 mg every 72 hours with increased monitoring of adverse events when administered concomitantly with FORTOVASE or FORTOVASE/ritonavir. Use tadalafil with caution at reduced doses of no more than 10 mg every 72 hours with increased monitoring of adverse events when administered concomitantly with FORTOVASE or FORTOVASE/ritonavir. |
| Tricyclic antidepressants:
Amitriptyline, imipramine | ↑ Tricyclics | Therapeutic concentration monitoring is recommended for tricyclic antidepressants when coadministered with FORTOVASE/ritonavir. |
Drugs That Are Mainly Metabolized by CYP3A4
Although specific studies have not been performed, coadministration with drugs that are mainly metabolized by CYP3A4 (eg, calcium channel blockers, dapsone, disopyramide, quinine, amiodarone, quinidine, warfarin, tacrolimus, cyclosporine, ergot derivatives, pimozide, carbamazepine, fentanyl, alfentanyl, alprazolam, nefazodone and triazolam) may have elevated plasma concentrations when coadministered with saquinavir; therefore, these combinations should be used with caution. If FORTOVASE is coadministered with ritonavir, the ritonavir label should be reviewed for additional drugs that should not be coadministered.
Inducers of CYP3A4
Coadministration with compounds that are potent inducers of CYP3A4 (eg, phenobarbital, phenytoin, dexamethasone, carbamazepine) may result in decreased plasma levels of saquinavir.
Carcinogenesis, Mutagenesis and Impairment Of Fertility
Carcinogenesis
Carcinogenicity studies found no indication of carcinogenic activity in rats and mice administered saquinavir for approximately 2 years. The plasma exposures (AUC values) in the respective species were up to approximately 60% of (using rat) and equivalent to (using mouse) those obtained in humans at the recommended clinical dose (FORTOVASE 1200 mg tid).
Mutagenesis
Mutagenicity and genotoxicity studies, with and without metabolic activation where appropriate, have shown that saquinavir has no mutagenic activity in vitro in either bacterial (Ames test) or mammalian cells (Chinese hamster lung V79/HPRT test). Saquinavir does not induce chromosomal damage in vivo in the mouse micronucleus assay or in vitro in human peripheral blood lymphocytes and does not induce primary DNA damage in vitro in the unscheduled DNA synthesis test.
Impairment of Fertility
Fertility and reproductive performance were not affected in rats at plasma exposures (AUC values) approximately 50% of those achieved in humans at the recommended dose.
Pregnancy
Teratogenic Effects
Category B. Reproduction studies conducted with saquinavir in rats have shown no embryotoxicity or teratogenicity at plasma exposures (AUC values) approximately 50% of those achieved in humans at the recommended dose or in rabbits at plasma exposures approximately 40% of those achieved at the recommended clinical dose of FORTOVASE. Distribution studies in these species showed that placental transfer of saquinavir is low (less than 5% of maternal plasma concentrations).
Studies in rats indicated that exposure to saquinavir from late pregnancy through lactation at plasma concentrations (AUC values) approximately 50% of those achieved in humans at the recommended dose of FORTOVASE had no effect on the survival, growth and development of offspring to weaning. Clinical experience in pregnant women is limited. Saquinavir should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Antiretroviral Pregnancy Registry
To monitor maternal-fetal outcomes of pregnant women exposed to antiretroviral medications, including FORTOVASE, an Antiretroviral Pregnancy Registry has been established. Physicians are encouraged to register patients by calling 1-800-258-4263.
Nursing Mothers
The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breast-feed their infants to avoid risking postnatal transmission of HIV. It is not known whether saquinavir is excreted in human milk. Because of both the potential for HIV transmission and the potential for serious adverse reactions in nursing infants, mothers should be instructed not to breast-feed if they are receiving antiretroviral medications, including FORTOVASE.
Pediatric Use
FORTOVASE should not be administered as a sole protease inhibitor to pediatric patients ≤16 years of age due to the risk of reduced saquinavir plasma concentrations compared to adults.
Safety and effectiveness of saquinavir when coadministered with ritonavir to pediatric patients is under investigation.
Geriatric Use
Clinical studies of FORTOVASE did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, caution should be taken when dosing FORTOVASE in elderly patients due to the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
(SEE PRECAUTIONS)
The safety of FORTOVASE was studied in more than 500 patients who received the drug either alone or in combination with other antiretroviral agents. The majority of treatment-related adverse events were of mild intensity. The most frequently reported treatment-emergent adverse events among patients receiving FORTOVASE in combination with other antiretroviral agents were diarrhea, nausea, abdominal discomfort, and dyspepsia.
Clinical adverse events of at least moderate intensity, which occurred in ≥2% of patients in studies NV15182 (an open-label, single-arm safety study) and NV15355 (an open-label randomized study comparing FORTOVASE and INVIRASE) are summarized in Table 7. The median duration of treatment in studies NV15182 and NV15355 were 52 and 18 weeks, respectively. In NV15182, more than 300 patients were on treatment for approximately 1 year.
FORTOVASE did not appear to alter the pattern, frequency or severity of known major toxicities associated with the use of nucleoside analogues. Physicians should refer to the complete product information for other antiretroviral agents as appropriate for drug-associated adverse reactions to these other agents.
Rare occurrences of the following serious adverse experiences have been reported during clinical trials of FORTOVASE and/or INVIRASE and were considered at least possibly related to use of study drugs: confusion, ataxia and weakness; seizures; headache; acute myeloblastic leukemia; hemolytic anemia; thrombocytopenia; thrombocytopenia and intracranial hemorrhage leading to death; attempted suicide; Stevens-Johnson syndrome; bullous skin eruption and polyarthritis; severe cutaneous reaction associated with increased liver function tests; isolated elevation of transaminases; exacerbation of chronic liver disease with Grade 4 elevated liver function tests, jaundice, ascites, and right and left upper quadrant abdominal pain; pancreatitis leading to death; intestinal obstruction; portal hypertension; thrombophlebitis; peripheral vasoconstriction; drug fever; nephrolithiasis; and acute renal insufficiency.
| NV15182 (48 weeks) | NV15355 (48 weeks) Naive Patients |
|
|---|---|---|
| ADVERSE EVENT | FORTOVASE + TOC†
N=442 | FORTOVASE+ 2 RTIs‡
N=90 |
| Gastrointestinal | ||
| Diarrhea | 19.9 | 15.6 |
| Nausea | 10.6 | 13.3 |
| Abdominal Discomfort | 8.6 | 10.0 |
| Dyspepsia | 8.4 | 7.8 |
| Flatulence | 5.7 | 10.0 |
| Vomiting | 2.9 | 4.4 |
| Abdoiminal Pain | 2.3 | 4.4 |
| Constipation | – | 3.3 |
| Body as a Whole | ||
| Fatigue | 4.8 | 8.9 |
| Appetite Decreased | – | 2.2 |
| Chest Pain | – | 2.2 |
| Central and Peripheral Nervous System | ||
| Headaches | ||
| 5.0 | 5.6 | |
| Psychiatric Disorders | ||
| Depression | 2.7 | – |
| Insomnia | – | 5.6 |
| Anxiety | – | 2.2 |
| Libido Disorder | – | 3.3 |
| Special Senses Disorders | ||
| Taste Alteration | – | 4.4 |
| Musculoskeletal Disorders | ||
| Pain | – | 3.3 |
| Dermatological Disorders | ||
| Eczema | – | – |
| Rash | – | – |
| Verruca | – | 2.2 |
Concomitant Therapy with Ritonavir
| FORTOVASE 1000 mg plus Ritonavir 100 mg bid (48 weeks) N=148 n(%=n/N) |
|
|---|---|
| Endocrine Disorders | |
| Diabetes mellitus/hyperglycemia | 4 (2.7) |
| Lipodystrophy | 8 (5.4) |
| Gastrointestinal Disorders | |
| Nausea | 16 (10.8) |
| Vomiting | 11 (7.4) |
| Diarrhea | 12 (8.1) |
| Abdominal Pain | 9 (6.1) |
| Constipation | 3 (2.0) |
| General Disorders and Administration Site Conditions | |
| Fatigue | 9 (6.1) |
| Fever | 5 (3.4) |
| Musculoskeletal Disorders | |
| Back Pain | 3 (2.0) |
| Respiratory Disorders | |
| Pneumonia | 8 (5.4) |
| Bronchitis | 4 (2.7) |
| Influenza | 4 (2.7) |
| Sinusitis | 4 (2.7) |
| Dermatological Disorders | |
| Rash | 5 (3.4) |
| Pruritus | 5 (3.4) |
| Dry lips/skin | 3 (2.0) |
| Eczema | 3 (2.0) |
Includes events with unknown relationship to study drug.
Laboratory Abnormalities
In the MaxCmin 1 study, Grade 3 and 4 thrombocytopenia (2.0% of patients) and anemia (2.0%) were observed with FORTOVASE in combination with ritonavir. At 48 weeks, other lab abnormalities included increased ALT, increased AST, increased GGT, hyperglycemia, hypertriglyceridemia, increased TSH, neutropenia, raised amylase, and increased LDH.
Table 9 summarizes the percentage of patients with marked laboratory abnormalities in study NV15182 and NV15355 (median duration of treatment was 52 and 18 weeks, respectively). In study NV15182, by 48 weeks <1% of patients discontinued treatment due to laboratory abnormalities.
In the safety study (NV15182), 27% to 33% of subjects experienced ≥1 grade shifts in ALT and AST during the 48-week study period. In 46% of such events, there was a single abnormal transaminase level with no evidence of persistently elevated enzyme values during the course of study. Only 3% to 4% of patients had ≥3 grade shifts in transaminase levels and less than 0.5% of patients had to discontinue the study for increased liver function test values.
| NV15182 (48 weeks) | NV15355 (48 weeks) Naive Patients |
||
|---|---|---|---|
| FORTOVASE + TOC†
N=442 | FORTOVASE + 2 RTIs‡
N=90 |
||
| ND Not done. | |||
| BIOCHEMISTRY | Limit | ||
| Alkaline Phosphatase (high) | >5 × ULN§ | 0.5 | 0.0 |
| Calcium (high) | >12.5 mg/dL | 0.2 | 0.0 |
| Creatine Kinase (high) | >4 × ULN§ | 7.8 | 6.0 |
| Gamma GT (high) | >5 × ULN§ | 5.7 | 5.0 |
| Glucose (low) | <40 mg/dL | 6.4 | 3.5 |
| Glucose (high) | >250 mg/dL | 1.4 | 0.0 |
| Phosphate (low) | <1.5 mg/dL | 0.5 | 1.0 |
| Potassium (high) | >6.5 mEq/L | 2.7 | 3.5 |
| Serum Amylase (high) | >2 × ULN§ | 1.9 | ND |
| SGOT (AST) (high) | >5 × ULN§ | 4.1 | 0.0 |
| SGPT (ALT) (high) | >5 × ULN§ | 5.7 | 1.0 |
| Sodium (high) | >157 mEq/L | 0.7 | 0.0 |
| Sodium (low) | <123 mEq/L | 0.0 | 1.0 |
| Total Bilirubin (high) | >2.5 × ULN§ | 1.6 | 0.0 |
| Triglycerides (high) | >750 mg/dL | 0.0 | 2.0 |
| HEMATOLOGY | |||
| Hemoglobin (low) | <7.0 gm/dL | 0.7 | 1.0 |
| Absolute Neutrophil Count (low) | <750 mm3 | 2.9 | 1.0 |
| Platelets (low) | <50,000 mm3 | 0.9 | 0.0 |
Additional marked lab abnormalities have been observed with INVIRASE. These include: calcium (low), phosphate (low), potassium (low), sodium (low).
Monotherapy and Combination Studies
Other clinical adverse experiences of any intensity, at least remotely related to FORTOVASE and INVIRASE, including those in <2% of patients, are listed below by body system.
Autonomic Nervous System
Mouth dry, night sweats, sweating increased
Body as a Whole
Allergic reaction, anorexia, appetite decreased, appetite disturbances, asthenia, chest pain, edema, fever, intoxication, malaise, olfactory disorder, pain body, pain pelvic, retrosternal pain, shivering, trauma, wasting syndrome, weakness generalized, weight decrease, redistribution/accumulation of body fat (see PRECAUTIONS: Fat Redistribution)
Cardiovascular/Cerebrovascular
Cyanosis, heart murmur, heart rate disorder, heart valve disorder, hypertension, hypotension, stroke, syncope, vein distended
Central and Peripheral Nervous System
Ataxia, cerebral hemorrhage, confusion, convulsions, dizziness, dysarthria, dysesthesia, hyperesthesia, hyperreflexia, hyporeflexia, light-headed feeling, myelopolyradiculoneuritis, neuropathy, numbness extremities, numbness face, paresis, paresthesis, peripheral neuropathy, poliomyelitis, prickly sensation, progressive multifocal leukoencephalopathy, spasms, tremor, unconsciousness
Dermatological
Acne, alopecia, chalazion, dermatitis, dermatitis seborrheic, erythema, folliculitis, furunculosis, hair changes, hot flushes, nail disorder, papillomatosis, papular rash, photosensitivity reaction, pigment changes skin, parasites external, pruritus, psoriasis, rash maculopapular, rash pruritic, red face, skin disorder, skin nodule, skin syndrome, skin ulceration, urticaria, verruca, xeroderma
Endocrine/Metabolic
Dehydration, diabetes mellitus, hyperglycemia, hypoglycemia, hypothyroidism, thirst, triglyceride increase, weight increase
Gastrointestinal
Abdominal distention, bowel movements frequent, buccal mucosa ulceration, canker sores oral, cheilitis, colic abdominal, dysphagia, esophageal ulceration, esophagitis, eructation, fecal incontinence, feces bloodstained, feces discolored, gastralgia, gastritis, gastroesophageal reflux, gastrointestinal inflammation, gingivitis, glossitis, hemorrhage rectum, hemorrhoids, infectious diarrhea, melena, painful defecation, parotid disorder, pruritus ani, pyrosis, salivary glands disorder, stomach upset, stomatitis, taste unpleasant, toothache, tooth disorder, ulcer gastrointestinal
Hematologic
Anemia, neutropenia, pancytopenia, splenomegaly
Liver and Biliary
Cholangitis sclerosing, cholelithiasis, hepatitis, hepatomegaly, hepatosplenomegaly, jaundice, liver enzyme disorder, pancreatitis
Musculoskeletal
Arthralgia, arthritis, back pain, cramps leg, cramps muscle, lumbago, musculoskeletal disorders, myalgia, myopathy, pain facial, pain jaw, pain leg, pain musculoskeletal, stiffness, tissue changes
Neoplasm
Kaposi's sarcoma, tumor
Platelet, Bleeding, Clotting
Bleeding dermal, hemorrhage, microhemorrhages, thrombocytopenia
Psychiatric
Agitation, amnesia, anxiety attack, behavior disturbances, dreaming excessive, euphoria, hallucination, intellectual ability reduced, irritability, lethargy, overdose effect, psychic disorder, psychosis, somnolence, speech disorder
Reproductive System
Epididymitis, erectile impotence, impotence, menstrual disorder, menstrual irregularity, penis disorder, prostate enlarged, vaginal discharge
Resistance Mechanism
Abscess, angina tonsillaris, candidiasis, cellulitis, herpes simplex, herpes zoster, infection bacterial, infection mycotic, infection staphylococcal, infestation parasitic, influenza, lymphadenopathy, molluscum contagiosum, moniliasis
Respiratory
Asthma bronchial, bronchitis, cough, dyspnea, epistaxis, hemoptysis, laryngitis, pharyngitis, pneumonia, pulmonary disease, respiratory disorder, rhinitis, rhinitis allergic atopic, sinusitis, upper respiratory tract infection
Special Senses
Blepharitis, conjunctivitis, cytomegalovirus retinitis, dry eye syndrome, earache, ear pressure, eye irritation, hearing decreased, otitis, taste unpleasant, tinnitus, visual disturbance, xerophthalmia
Urinary System
Micturition disorder, nocturia, renal calculus, renal colic, urinary tract bleeding, urinary tract infection
Postmarketing Experience with INVIRASE and FORTOVASE
Additional adverse events that have been observed during the postmarketing period are similar to those seen in clinical trials with INVIRASE and FORTOVASE and administration of INVIRASE and FORTOVASE in combination with ritonavir.
OVERDOSAGE
Two cases of FORTOVASE overdosage have been received (one case with unknown amount of FORTOVASE, the second case 3.6 to 4 grams at once). No adverse events have been reported in both cases. There were 2 patients who had overdoses with INVIRASE. No acute toxicities or sequelae were noted in the first patient after ingesting 8 grams of INVIRASE as a single dose. The patient was treated with induction of emesis within 2 to 4 hours after ingestion. The second patient ingested 2.4 grams of INVIRASE in combination with 600 mg of ritonavir and experienced pain in the throat that lasted for 6 hours and then resolved.
DOSAGE AND ADMINISTRATION
FORTOVASE (saquinavir) soft gelatin capsules and INVIRASE (saquinavir mesylate) capsules are not bioequivalent and cannot be used interchangeably. When using saquinavir as the sole protease inhibitor in an antiviral regimen, FORTOVASE is the recommended formulation. INVIRASE may be considered only if it is combined with ritonavir, which significantly inhibits saquinavir's metabolism to provide plasma saquinavir levels at least equal to those achieved with FORTOVASE at the recommended dose of 1200 mg tid (see CLINICAL PHARMACOLOGY: Drug Interactions).
Adults (Over the Age of 16 Years)
FORTOVASE Administered Without Ritonavir:
- FORTOVASE 1200-mg tid (6 × 200-mg capsules)
- FORTOVASE should be taken with a meal or up to 2 hours after a meal
FORTOVASE Administered With Ritonavir:
- FORTOVASE 1000-mg bid (5 × 200-mg capsules) in combination with ritonavir 100-mg bid
- Ritonavir should be taken at the same time as FORTOVASE
- FORTOVASE and ritonavir should be taken within 2 hours after a meal
When used in combination with nucleoside analogues, the dosage of FORTOVASE should not be reduced as this will lead to greater than dose proportional decreases in saquinavir plasma levels.
Patients should be advised that FORTOVASE, like other protease inhibitors, is recommended for use in combination with active antiretroviral therapy. Greater activity has been observed when new antiretroviral therapies are begun at the same time as FORTOVASE. As with all protease inhibitors, adherence to the prescribed regimen is strongly recommended. Concomitant therapy should be based on a patient's prior drug exposure.
Monitoring of Patients
Clinical chemistry tests, viral load, and CD4 count should be performed prior to initiating FORTOVASE therapy and at appropriate intervals thereafter. For comprehensive patient monitoring recommendations for other antiretroviral therapies, physicians should refer to the complete product information for these drugs.
Dose Adjustment for Combination Therapy with FORTOVASE
For serious toxicities that may be associated with FORTOVASE, the drug should be interrupted. For recipients of combination therapy with FORTOVASE and other antiretroviral agents, dose adjustment of the other antiretroviral agents should be based on the known toxicity profile of the individual drug. FORTOVASE dose adjustments may be required with some other antiretroviral agents (see PRECAUTIONS: Drug Interactions). Physicians should refer to the complete product information for these drugs for comprehensive dose adjustment recommendations and drug-associated adverse reactions.
HOW SUPPLIED
FORTOVASE 200-mg capsules are beige, opaque, soft gelatin capsules with ROCHE and 0246 imprinted on the capsule shell — bottles of 180 (NDC 0004-0246-48).
The capsules should be refrigerated at 36° to 46°F (2° to 8°C) in tightly closed bottles until dispensed.
For patient use, refrigerated (36° to 46°F, 2° to 8°C) capsules of FORTOVASE remain stable until the expiration date printed on the label. Once brought to room temperature [at or below 77°F (25°C)], capsules should be used within 3 months.
HIVID and INVIRASE are registered trademarks of Hoffmann-La Roche Inc. KALETRA is a registered trademark of Abbott Laboratories.
Manufactured by:
F. Hoffmann-La Roche Ltd., Basel, Switzerland
Distributed by:

27898696
Copyright © 1997-2003 by Roche Laboratories Inc. All rights reserved.
| Fortovase (Saquinavir) | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
Revised: 04/2006