FUSILEV
-
levoleucovorin calcium injection, powder, lyophilized, for solution
Spectrum Pharmaceuticals, Inc.
----------
FUSILEV These highlights do not include all the information needed to use Fusilev safely and effectively. See full prescribing information for Fusilev. FUSILEV (levoleucovorin calcium) powder, injection, lyophilized, for solution for intravenous use Initial U.S. Approval: 1952 (d,l-leucovorin), 2008 (levoleucovorin)Fusilev is a folate analog. (1)
Fusilev rescue is indicated after high-dose methotrexate therapy in osteosarcoma. (1)
Fusilev is also indicated to diminish the toxicity and counteract the effects of impaired methotrexate elimination and of inadvertent overdosage of folic acid antagonists. (1)
Limitations of Use
Fusilev is not approved for pernicious anemia and megaloblastic anemias. Improper use may cause a hematologic remission while neurologic manifestations continue to progress. (1.1)
Fusilev Rescue After High-Dose Methotrexate Therapy
Do not administer intrathecally. (2.1)
Fusilev is dosed at one-half the usual dose of the racemic form. (2.1)
Fusilev rescue recommendations are based on a methotrexate dose of 12 grams/msuperscript 2 administered by intravenous infusion over 4 hours. Fusilev rescue at a dose of 7.5 mg (approximately 5 mg/m superscript 2) every 6 hours for 10 doses starts 24 hours after the beginning of the methotrexate infusion. Determine serum creatinine and methotrexate levels at least once daily. Continue Fusilev administration, hydration, and urinary alkalinization (pH of 7.0 or greater) until the methotrexate level is below 5 x 10 superscript -8M (0.05 micromolar). The Fusilev dose may need to be adjusted. (2.3)
Each 50 mg single-use vial of Fusilev contains a sterile lyophilized powder consisting of 64 mg levoleucovorin calcium pentahydrate (equivalent to 50 mg levoleucovorin ) and 50 mg mannitol. (16) It is intended for intravenous administration after reconstitution with 5.3 mL of sterile 0.9 percent Sodium Chloride for Injection, USP. (2.5, 11)
Fusilev is contraindicated for patients who have had previous allergic reactions attributed to folic acid or folinic acid. (4)
Due to Ca superscript ++ content, no more than 16 mL (160 mg) of levoleucovorin solution should be injected intravenously per minute. (5.1)Fusilev enhances the toxicity of fluorouracil. (5.2,7)
Concomitant use of d,l-leucovorin with trimethoprim-sulfamethoxazole for Pneumocystis carinii pneumonia in HIV patients was associated with increased rates of treatment failure in a placebo-controlled study. (5.3)
Allergic reactions were reported in patients receiving Fusilev. (6.2)
Vomiting (38 percent), stomatitis (38 percent) and nausea (19 percent) were reported in patients receiving Fusilev as rescue after high dose methotrexate therapy. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Spectrum Pharmaceuticals, Inc. at 1-877-387-4538 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Fusilev may counteract the antiepileptic effect of phenobarbital, phenytoin and primidone, and increase the frequency of seizures in susceptible patients. (7)
FULL PRESCRIBING INFORMATION: CONTENTS
1. INDICATIONS AND USAGE1.1 Limitations of Use
2. DOSAGE AND ADMINISTRATION
2.1 Administration Guidelines
2.2 Co-administration of Fusilev with other agents
2.3 Fusilev Rescue After High-Dose Methotrexate Therapy
2.4 Dosing Recommendations for Inadvertent Methotrexate Overdosage
2.5 Reconstitution and Infusion Instructions
3. DOSAGE FORMS AND STRENGTHS
4. CONTRAINDICATIONS
5. WARNINGS AND PRECAUTIONS
5.1 Rate of Administration
5.2 Potential for Enhanced Toxicity with 5-Fluorouracil
5.3 Potential for interaction with trimethoprim-sulfamethoxazole
6. ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
7. DRUG INTERACTIONS
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10. OVERDOSAGE
11. DESCRIPTION
12. CLINICAL PHARMACOLOGY
12.1 Mechanism Of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment Of Fertility
13.2 Animal Toxicology And/Or Pharmacology
14. CLINICAL STUDIES
16. HOW SUPPLIED/STORAGE AND HANDLING
Sections or subsections omitted from the full prescribing information are not listed
1. INDICATIONS AND USAGE
Fusilev™ is a folate analog.
Fusilev rescue is indicated after high-dose methotrexate therapy in osteosarcoma.
Fusilev is also indicated to diminish the toxicity and counteract the effects of impaired methotrexate elimination and of inadvertent overdosage of folic acid antagonists.
1.1 Limitations of Use
Fusilev is not approved for pernicious anemia and megaloblastic anemias secondary to the lack of vitamin B12. Improper use may cause a hematologic remission while neurologic manifestations continue to progress
2.1 Administration Guidelines
Fusilev is dosed at one-half the usual dose of the racemic form.
Fusilev is indicated for intravenous administration only. Do not administer intrathecally.
2.2 Co-administration of Fusilev with other agents
Due to the risk of precipitation, do not co-administer Fusilev with other agents in the same admixture.
2.3 Fusilev Rescue After High-Dose Methotrexate Therapy
The recommendations for Fusilev rescue are based on a methotrexate dose of 12 grams/m2 administered by intravenous infusion over 4 hours (see methotrexate package insert for full prescribing information). Fusilev rescue at a dose of 7.5 mg (approximately 5 mg/m2) every 6 hours for 10 doses starts 24 hours after the beginning of the methotrexate infusion.
Serum creatinine and methotrexate levels should be determined at least once daily. Fusilev administration, hydration, and urinary alkalinization (pH of 7.0 or greater) should be continued until the methotrexate level is below 5 x 10-8 M (0.05 micromolar). The Fusilev dose should be adjusted or rescue extended based on the following guidelines
| Clinical Situation | Laboratory Findings | Fusileve Dosage and Duration |
|---|---|---|
| Normal Methotrexate Elimination | Serum methotrexate level approximately 10 micromolar at 24 hours after administration, 1 micromolar at 48 hours, and less than 0.2 micromolar at 72 hours | 7.5 mg IV q 6 hours for 60 hours (10 doses starting at 24 hours after start of methotrexate infusion). |
| Delayed Late Methotrexate Elimination | Serum methotrexate level remaining above 0.2 micromolar at 72 hours, and more than 0.05 micromolar at 96 hours after administration. | Continue 7.5 mg IV q 6 hours, until methotrexate level is less than 0.05 micromolar. |
| Delayed Early Methotrexate Elimination and/or Evidence of Acute Renal Injury | Serum methotrexate level of 50 micromolar or more at 24 hours, or 5 micromolar or more at 48 hours after administration, OR; a 100 percent or greater increase in serum creatinine level at 24 hours after methotrexate administration (e.g., an increase from 0.5 mg/dL to a level of 1 mg/dL or more). | 75 mg IV q 3 hours until methotrexate level is less than 1 micromolar; then 7.5 mg IV q 3 hours until methotrexate level is less than 0.05 micromolar. |
Some patients will have abnormalities in methotrexate elimination or renal function following methotrexate administration, which are significant but less severe than the abnormalities described in the table above. These abnormalities may or may not be associated with significant clinical toxicity. If significant clinical toxicity is observed, Fusilev rescue should be extended for an additional 24 hours (total of 14 doses over 84 hours) in subsequent courses of therapy. The possibility that the patient is taking other medications which interact with methotrexate (e.g., medications which may interfere with methotrexate elimination or binding to serum albumin) should always be reconsidered when laboratory abnormalities or clinical toxicities are observed.
Delayed methotrexate excretion may be caused by accumulation in a third space fluid collection (i.e., ascites, pleural effusion), renal insufficiency, or inadequate hydration. Under such circumstances, higher doses of Fusilev or prolonged administration may be indicated.
Although Fusilev may ameliorate the hematologic toxicity associated with high dose methotrexate, Fusilev has no effect on other established toxicities of methotrexate such as the nephrotoxicity resulting from drug and/or metabolite precipitation in the kidney.
2.4 Dosing Recommendations for Inadvertent Methotrexate Overdosage
Fusilev rescue should begin as soon as possible after an inadvertent overdosage and within 24 hours of methotrexate administration when there is delayed excretion. As the time interval between antifolate administration [e.g., methotrexate] and Fusilev rescue increases, Fusilev’s effectiveness in counteracting toxicity may decrease. Fusilev 7.5 mg (approximately 5 mg/m2 ) should be administered IV every 6 hours until the serum methotrexate level is less than 10-8 M.
Serum creatinine and methotrexate levels should be determined at 24 hour intervals. If the 24 hour serum creatinine has increased 50% over baseline or if the 24 hour methotrexate level is greater than 5 x 10-6 M or the 48 hour level is greater than 9 x 10-7 M, the dose of Fusilev should be increased to 50 mg/m2 IV every 3 hours until the methotrexate level is less than 10-8 M. Hydration (3 L/day) and urinary alkalinization with NaHCO3 should be employed concomitantly. The bicarbonate dose should be adjusted to maintain the urine pH at 7.0 or greater.
2.5 Reconstitution and Infusion Instructions
Prior to intravenous injection, the 50 mg vial of Fusilev for Injection is reconstituted with 5.3 mL of 0.9% Sodium Chloride Injection, USP to yield a levoleucovorin concentration of 10 mg per mL. Reconstitution with Sodium Chloride solutions with preservatives (e.g. benzyl alcohol) has not been studied. The use of solutions other than 0.9 percent Sodium Chloride Injection, USP is not recommended.
The reconstituted 10 mg per mL levoleucovorin contains no preservative. Observe strict aseptic technique during reconstitution of the drug product.
Saline reconstituted levoleucovorin solutions may be further diluted, immediately, to concentrations of 0.5 mg/mL to 5 mg/mL in 0.9 percent Sodium Chloride Injection, USP or 5 percent Dextrose Injection, USP. Initial reconstitution or further dilution using 0.9 percent Sodium Chloride Injection, USP may be held at room temperature for not more than a total of 12 hours. Dilutions in 5 percent Dextrose Injection, USP may be held at room temperature for not more than 4 hours.
Visually inspect the reconstituted solution for particulate matter and discoloration, prior to administration. CAUTION: Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if cloudiness or precipitate is observed.
No more than 16 mL of reconstituted solutions (160 mg of levoleucovorin ) should be injected intravenously per minute, because of the calcium content of the levoleucovorin solution.
3. DOSAGE FORMS AND STRENGTHS
Fusilev is supplied in sterile, single-use vials containing 64 mg levoleucovorin calcium pentahydrate (equivalent to 50 mg levoleucovorin ) and 50 mg mannitol.
Fusilev is contraindicated for patients who have had previous allergic reactions attributed to folic acid or folinic acid.
5. WARNINGS AND PRECAUTIONS
5.1 Rate of Administration
Because of the Ca++ content of the levoleucovorin solution, no more than 16 mL (160 mg of levoleucovorin ) should be injected intravenously per minute.
5.2 Potential for Enhanced Toxicity with 5-Fluorouracil
Fusilev enhances the toxicity of 5-fluorouracil. Deaths from severe enterocolitis, diarrhea, and dehydration have been reported in elderly patients receiving weekly d,l-leucovorin and 5-fluorouracil.
5.3 Potential for interaction with trimethoprim-sulfamethoxazole
The concomitant use of d,l-leucovorin with trimethoprim-sulfamethoxazole for the acute treatment of Pneumocystis carinii pneumonia in patients with HIV infection was associated with increased rates of treatment failure and morbidity in a placebo-controlled study.
6. ADVERSE REACTIONS
6.1 Clinical Studies Experience
Since clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The following table presents the frequency of adverse reactions which occurred during the administration of 58 courses of high dose methotrexate 12 grams/m2 followed by Fusilev rescue for osteosarcoma in 16 patients age 6-21. Most patients received Fusilev 7.5 mg every 6 hours for 60 hours or longer beginning 24 hours after completion of methotrexate.
| Body System/Adverse Reactions |
| Number (%) of Patients with Adverse Reactions |
| Number (%) of Courses with Adverse Reactions |
|---|---|---|---|---|
|
|
| (N =16) |
| (N = 58) |
|
| All | Grade 3 Plus | All | Grade 3 Plus |
| Gastrointestinal |
|
|
|
|
| Stomatitis | 6 (37.5) | 1 (6.3) | 10 (17.2) | 1 (1.7) |
| Vomiting | 6 (37.5) | 0 | 14 (24.1) | 0 |
| Nausea | 3 (18.8) | 0 | 3 (5.2) | 0 |
| Diarrhea | 1 (6.3) | 0 | 1 (1.7) | 0 |
| Dyspepsia | 1 (6.3) | 0 | 1 (1.7) | 0 |
| Typlitis | 1 (6.3) | 1 (6.3) | 1 (1.7) | 1 (1.7) |
| Respiratory |
|
|
|
|
| Dyspnea | 1 (6.3) | 0 | 1 (1.7) | 0 |
| Skin and Appendages |
|
|
|
|
| Dermatitis | 1 (6.3) | 0 | 1 (1.7) | 0 |
| Other |
|
|
|
|
| Confusion | 1 (6.3) | 0 | 1 (1.7) | 0 |
| Neuropathy | 1 (6.3) | 0 | 1 (1.7) | 0 |
| Renal function abnormal | 1 (6.3) | 0 | 3 (5.2) | 0 |
| Taste perversion | 1 (6.3) | 0 | 1 (1.7) | 0 |
|
|
|
|
|
|
| Total number of patients |
| 9 (56.3) |
| 2 (12.5) |
| Total number of courses |
| 25 (43.1) |
| 2 (3.4) |
The incidence of adverse reactions may be underestimated because not all patients were fully evaluable for toxicity for all cycles in the clinical trials. Leukopenia and thrombocytopenia were observed, but could not be attributed to high dose methotrexate with Fusilev rescue because patients were receiving other myelosuppressive chemotherapy.
6.2 Postmarketing Experience
Since adverse reactions from spontaneous reports are provided voluntarily from a population of uncertain size, it is not always possible to estimate reliably their frequency or establish a causal relationship to drug exposure. Spontaneously reported adverse reactions collected by the WHO Collaborating Center for International Drug Monitoring in Uppsala Sweden have yielded seven cases where levoleucovorin was administered with a regimen of methotrexate. The events were dyspnea, pruritus, rash, temperature change and rigors. For 217 adverse reactions (108 reports) where levoleucovorin was a suspected or interacting medication, there were 40 occurrences of “possible allergic reaction.”
7. DRUG INTERACTIONS
Folic acid in large amounts may counteract the antiepileptic effect of phenobarbital, phenytoin and primidone, and increase the frequency of seizures in susceptible children. It is not known whether folinic acid has the same effects. However, both folic and folinic acids share some common metabolic pathways. Caution should be taken when taking folinic acid in combination with anticonvulsant drugs.
Preliminary human studies have shown that small quantities of systemically administered leucovorin enter the CSF, primarily as its major metabolite, 5-methyltetrahydrofolate (5-MTHFA). In humans, the CSF levels of 5-MTHFA remain 1-3 orders of magnitude lower than the usual methotrexate concentrations following intrathecal administration.
Fusilev increases the toxicity of 5-fluorouracil [see Warnings and Precautions (5.2)].
8. USE IN SPECIFIC POPULATIONS
8.1 PregnancyPregnancy Category C. It is not known whether Fusilev can cause fetal harm when administered to a pregnant woman or if it can affect reproduction capacity. Animal reproduction studies have not been conducted with Fusilev. Fusilev should be given to a pregnant woman only if clearly needed.
8.3 Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Fusilev is administered to a nursing mother
8.4 Pediatric Use
[See Clinical Studies (14)]
8.5 Geriatric Use
Clinical studies of Fusilev in the treatment of osteosarcoma did not include subjects aged 65 and over to determine whether they respond differently from younger subjects.
10. OVERDOSAGE
No data are available for overdosage with levoleucovorin
11. DESCRIPTION
Levoleucovorin is the levo isomeric form of racemic d,l-leucovorin, present as the calcium salt. Levoleucovorin is the pharmacologically active isomer of leucovorin [(6-S)-leucovorin].
Fusilev for injection contains levoleucovorin calcium, which is one of several active, chemically reduced derivatives of folic acid. It is useful as antidote to the inhibition of dihydrofolate reductase by methotrexate. This compound has the chemical designation calcium (6S)-N-{4-[[(2-amino-5-formyl-1,4,5,6,7,8-hexahydro-4-oxo-6-pteridinyl)methyl] amino]benzoyl}-L-glutamate pentahydrate. The molecular weight is 601.6 and the structural formula is:
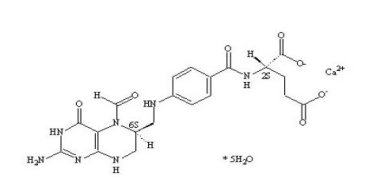
Fusilev for injection is supplied as a sterile lyophilized powder consisting of 64 mg levoleucovorin calcium pentahydrate (equivalent to 50 mg levoleucovorin) and 50 mg mannitol per 50 mg vial.
Sodium hydroxide and/or hydrocholoric acid are used to adjust the pH during manufacture. It is intended for intravenous administration after reconstitution with 5.3 mL of sterile 0.9 percent Sodium Chloride Injection, USP [See Dosage and Administration (2.5)]
12. CLINICAL PHARMACOLOGY
12.1 Mechanism Of Action
Levoleucovorin is the pharmacologically active isomer of 5-formyl tetrahydrofolic acid. Levoleucovorin does not require reduction by the enzyme dihydrofolate reductase in order to participate in reactions utilizing folates as a source of “one-carbon” moieties. Administration of levoleucovorin can counteract the therapeutic and toxic effects of folic acid antagonists such as methotrexate, which act by inhibiting dihydrofolate reductase.
12.2 Pharmacodynamics
Levoleucovorin is actively and passively transported across cell membranes. In vivo, levoleucovorin is converted to 5-methyltetrahydrofolic acid (5-methyl-THF), the primary circulating form of active reduced folate. Levoleucovorin and 5-methyl-THF are polyglutamated intracellularly by the enzyme folylpolyglutamate synthetase. Folylpolyglutamates are active and participate in biochemical pathways that require reduced folate.
12.3 Pharmacokinetics
The pharmacokinetics of levoleucovorin after intravenous administration of a 15 mg dose was studied in healthy male volunteers. After rapid intravenous administration, serum total tetrahydrofolate (total-THF) concentrations reached a mean peak of 1722 ng/mL. Serum (6S)-5-methyl-5,6,7,8-tetrahydrofolate concentrations reached a mean peak of 275 ng/mL and the mean time to peak was 0.9 hours. The mean terminal half-life for total-THF and (6S)-5-methyl-5,6,7,8-tetrahydrofolate was 5.1 and 6.8 hours, respectively
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment Of Fertility
No studies have been conducted to evaluate the potential of levoleucovorin for carcinogenesis, mutagenesis and impairment of fertility.
13.2 Animal Toxicology And/Or Pharmacology
The acute intravenous LD50 values in adult mice and rats were 575 mg/kg (1725 mg/m2) and 378 mg/kg ( 2268 mg/m2), respectively. Signs of sedation, tremors, reduced motor activity, prostration, labored breathing, and/or convulsion were observed in these studies. Anticipated human dose for each administration is approximately 5 mg/m2, which represents a 3-log safety margin.
14. CLINICAL STUDIES
The safety and efficacy of Fusilev rescue following high-dose methotrexate were evaluated in 16 patients age 6-21 who received 58 courses of therapy for osteogenic sarcoma. High-dose methotrexate was one component of several different combination chemotherapy regimens evaluated across several trials. Methotrexate 12 g/m2 IV over 4 hours was administered to 13 patients, who received Fusilev 7.5 mg every 6 hours for 60 hours or longer beginning 24 hours after completion of methotrexate. Three patients received methotrexate 12.5 g/m2 IV over 6 hours, followed by Fusilev 7.5 mg every 3 hours for 18 doses beginning 12 hours after completion of methotrexate. The mean number of Fusilev doses per course was 18.2 and the mean total dose per course was 350 mg. The efficacy of Fusilev rescue following high-dose methotrexate was based on the adverse reaction profile. [See Adverse Reactions (6)]
16. HOW SUPPLIED/STORAGE AND HANDLING
Each 50 mg single-use vial of Fusilev for Injection contains a sterile lyophilized powder consisting of 64 mg levoleucovorin calcium pentahydrate (equivalent to 50 mg levoleucovorin) and 50 mg mannitol.
50 mg vial of freeze-dried powder – NDC 68152-101-00.
Store at 25 degrees C (77 degrees F) in carton until contents are used. Excursions permitted from 15-30 degrees C (59-86 degrees F). [See USP Controlled Room Temperature]. Protect from light.
| FUSILEV
levoleucovorin calcium injection, powder, lyophilized, for solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA020140 | 08/15/2008 | 02/24/2010 |
| Labeler - Spectrum Pharmaceuticals, Inc. (790888002) |
| Registrant - Cangene BioPharma Inc. (050783398) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Cangene BioPharma Inc. | 050783398 | manufacture | |