LIDOCAINE HYDROCHLORIDE AND DEXTROSE
-
lidocaine hydrochloride injection, solution
Hospira, Inc.
----------
0.4% and 0.8% LIDOCAINE HYDROCHLORIDE and 5% DEXTROSE INJECTION, USPAQUEOUS SOLUTIONS FOR ACUTE MANAGEMENT OF CARDIAC ARRHYTHMIAS
Flexible Plastic Container
Rx only
DESCRIPTION
0.4% and 0.8% Lidocaine Hydrochloride and 5% Dextrose Injection, USP are sterile, nonpyrogenic solutions of lidocaine hydrochloride and 5% Dextrose Injection, USP for use in the management of ventricular arrhythmias.
Each 100 mL contains lidocaine hydrochloride, anhydrous 400 mg (4 mg/mL) or 800 mg (8 mg/mL) and dextrose hydrous 5 g in water for injection. The osmolarity of the solutions is 282 and 311 mOsmol/liter (calc.) respectively. May contain hydrochloric acid and/or sodium hydroxide for pH adjustment. pH 4.0 (3.0 − 5.5).
The solution contains no bacteriostatic, antimicrobial agent or added buffer (except for pH adjustment) and is intended only for use as a single-dose administration. When smaller doses are required, the unused portion should be discarded.
Lidocaine administered intravenously is a cardiac antiarrhythmic agent.
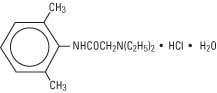
Lidocaine Hydrochloride, USP is chemically designated 2(diethylamino)2’, 6'-acetoxylidide monohydrochloride monohydrate, a white powder freely soluble in water. It has the following structural formula:
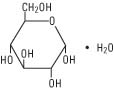
Dextrose, USP is chemically designated D-glucose monohydrate (C6H12O6•H2O), a hexose sugar freely soluble in water. It has the following structural formula:
Water for Injection, USP is chemically designated H2O.
The flexible plastic container is fabricated from a specially formulated polyvinylchloride. Water can permeate from inside the container into the overwrap but not in amounts sufficient to affect the solution significantly. Solutions inside the plastic container also can leach out certain of its chemical components in very small amounts before the expiration period is attained. However, the safety of the plastic has been confirmed by tests in animals according to USP biological standards for plastic containers.
CLINICAL PHARMACOLOGY
Intravenous injection of lidocaine hydrochloride produces very rapid antiarrhythmic actions. The antiarrhythmic effect quickly decreases once the infusion is stopped. Thus, ventricular ectopic activity can be titrated from one moment to the next.
Lidocaine reportedly exerts a cardiac antiarrhythmic effect by increasing the electrical stimulation threshold of the ventricle during diastole. At usual doses, lidocaine produces no change in myocardial contractility, in systemic arterial pressure or in absolute refractory period.
About 90% of an administered dose of the drug is metabolized in the liver. The remaining 10% is excreted unchanged in the urine.
Toxicity is related to lidocaine blood levels. The decreased clearance and longer half-life of lidocaine should be taken into consideration with prolonged (24 hour) infusions. Constant rate of infusion may result in toxic accumulation of lidocaine. Infusion should be reduced to approximately one half to compensate for decreased rate of clearance and concomitant or prior administration of propranolol may further increase blood concentrations by as much as 30% in patients without cardiac or hepatic failure. In clinical studies, patients over 65 years showed decreased lidocaine clearance. This was partly due to the tendency of elderly patients to have lower body weight and the increased risk of cardiac failure in these patients.
Solutions containing carbohydrate in the form of dextrose restore blood glucose levels and provide calories. Carbohydrate in the form of dextrose may aid in minimizing liver glycogen depletion and exerts a protein-sparing action. Dextrose injected parenterally undergoes oxidation to carbon dioxide and water.
Water is an essential constituent of all body tissues and accounts for approximately 70% of total body weight. Average normal adult daily requirements range from two to three liters (1 to 1.5 liters each for insensible water loss by perspiration and urine production).
Water balance is maintained by various regulatory mechanisms. Water distribution depends primarily on the concentration of electrolytes in the body compartments and sodium (Na+) plays a major role in maintaining physiologic equilibrium.
INDICATIONS AND USAGE
Lidocaine Hydrochloride administered intravenously is specifically indicated in the acute management of (1) ventricular arrhythmias occurring during cardiac manipulation, such as cardiac surgery; and (2) life-threatening arrhythmias, particularly those which are ventricular in origin, such as occur during acute myocardial infarction.
CONTRAINDICATIONS
Lidocaine is contraindicated in patients with a known hypersensitivity to local anesthetics of the amide type. Administration of lidocaine is contraindicated in patients with Adams-Stokes syndrome or with severe degrees of sinoatrial, atrioventricular or intraventricular heart block.
Dextrose solutions without electrolytes should not be administered simultaneously with blood through the same infusion set because of the possibility that pseudoagglutination of red cells may occur.
WARNINGS
Constant monitoring with an electrocardiograph is essential for proper administration of lidocaine. Signs of excessive depression of cardiac conductivity, such as prolongation of PR interval and QRS complex and appearance or aggravation of arrhythmias, should be followed by prompt cessation of intravenous infusion of this agent. It is mandatory to have emergency equipment and drugs immediately available to manage possible adverse reactions involving the cardiovascular, respiratory or central nervous systems.
Excess administration of potassium-free solutions may result in significant hypokalemia.
The intravenous administration of these solutions can cause fluid and/or solute overloading resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema.
As the dosage of dilute solutions of lidocaine must be titrated to individual patient response, additive medications should not be delivered via this solution. (See PRECAUTIONS.)
Overt or relative toxicity may result in evidence of central nervous system depression (sedation) or irritability (twitching) which may progress to frank convulsions accompanied by respiratory depression and/or arrest. Early recognition of premonitory signs, assurance of adequate oxygenation and where necessary, establishment of artificial airway with ventilatory support are essential to management of this problem. Should convulsions persist despite ventilatory therapy, small increments of anticonvulsant drugs may be used intravenously. Examples of such agents include benzodiazepines (e.g., diazepam), and ultrashort-acting barbiturate (e.g., thiopental or thiamylal). If the patient is under anesthesia, a short-acting muscle relaxant (succinylcholine) may be used. Longer acting drugs should be used only when recurrent convulsions are evidenced.
PRECAUTIONS
The safe use of lidocaine requires careful electrocardiographic (ECG) observation in an environment and by persons capable of resuscitation.
Caution should be exercised with repeated use of lidocaine in patients with severe liver or renal disease because accumulation may lead to toxic phenomena, since the drug is metabolized mainly in the liver and partially excreted unchanged by the kidney. In patients with cardiac or hepatic failure, the rate of lidocaine clearance may be decreased. Prolonged (24 hour) infusion of the drug also appears to result in a longer half-life and reduced rate of clearance (even in patients without cardiac or hepatic failure) that may result in toxic accumulation of lidocaine in the plasma. After the first 24 hours, the rate of infusion of lidocaine should be reduced by about one-half to compensate for the drop in the rate of elimination of the drug.
Miscellaneous: Amide local anesthetic administration may be associated with acute onset of fulminant hypermetabolism of skeletal muscle known as malignant hyperthermic crisis. Key to successful outcome of fulminant hypermetabolism is early recognition of premonitory signs, i.e., tachycardia and increased metabolic rate as evidenced by respiratory and/or metabolic acidosis. Treatment includes administration of oxygen and discontinuation of infusion and, where necessary, intravenous administration of dantrolene sodium. For additional information on management see prescribing information of dantrolene sodium.
In patients with sinus bradycardia the intravenous administration of lidocaine for the elimination of ventricular ectopic beats without prior acceleration in heart rate (e.g., by isoproterenol or by electric pacing) may provoke more frequent and serious ventricular arrhythmias.
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations and acid-base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation.
Solutions containing dextrose should be used with caution in patients with known subclinical or overt diabetes mellitus.
Do not administer unless solution is clear and container is undamaged. Discard unused portion.
Pregnancy Category B.
Reproduction studies have been performed in rats at doses up to 5 times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to lidocaine hydrochloride. There are, however, no adequate well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pediatric Use:
The safety and effectiveness of lidocaine has not been established in pediatric patients (neonates to adolescents).
Geriatric Use:
Clinical studies of Lidocaine HCl did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Lidocaine is substantially excreted by the kidney and clearance of lidocaine is decreased in the elderly (see CLINICAL PHARMACOLOGY). In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased renal or cardiac function, and of concomitant disease or other drug therapy.
Drug Interactions
Lidocaine should be used with caution in patients with digitalis toxicity accompanied by atrioventricular block. Coadministration of propranolol with lidocaine has been reported to reduce the clearance of lidocaine from the plasma and may result in toxic accumulation of the drug.
When lidocaine is administered with other antiarrhythmic drugs such as phenytoin, procainamide, propranolol or quinidine, the cardiac effects may be additive or antagonistic and toxic effects may be additive. Phenytoin may stimulate the hepatic metabolism of lidocaine, but the clinical significance of this effect is not known.
ADVERSE REACTIONS
Adverse reactions are the result of systemic toxicity which affects primarily the central nervous system and the cardiovascular system.
Systemic reactions of the following types have been reported:
-
Central Nervous System: Light-headedness, drowsiness, dizziness, apprehension, euphoria, tinnitus, blurred or double vision, vomiting, sensation of heat, cold or numbness, twitching, tremors, convulsions, unconsciousness and respiratory depression and arrest.
-
Cardiovascular System: Hypotension; cardiovascular collapse and bradycardia which may lead to cardiac arrest. In the event of cardiac arrest, standard methods of cardiopulmonary resuscitation should be employed.
Allergic reactions are rare and may occur as a result of sensitivity to lidocaine and are characterized by cutaneous lesions of delayed onset, urticaria, edema or anaphylactoid symptoms. The detection of sensitivity by skin testing is of limited value.
There have been no reports of cross-sensitivity between lidocaine and procainamide or between lidocaine and quinidine.
Other reactions which may occur because of the solutions or technique of administration include febrile response (see PRECAUTIONS section on hyperthermia), infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia.
MANAGEMENT OF ADVERSE REACTIONS
In case of severe reaction, discontinue use of the drug.
Treatment of a patient with toxic manifestations consists of assuring and maintaining a patent airway and supporting ventilation with oxygen and assisted or controlled respiration as required. This usually will be sufficient in the management of most reactions. Should a convulsion persist despite oxygen therapy, small increments of an anticonvulsive agent may be given intravenously, such as a benzodiazepine (e.g., diazepam) or ultrashort-acting barbiturate (thiopental or thiamylal) or a short-acting barbiturate (pentobarbital or secobarbital).
Should circulatory depression occur, vasopressors, such as epinephrine, ephedrine or metaraminol, and intravenous fluids may be used and, when necessary, standard methods of cardiopulmonary resuscitation should be employed.
Allergic reactions should be managed by conventional means.
OVERDOSAGE
Reported adverse reactions are due to drug overdosage. (See ADVERSE REACTIONS section.) For management of overdosage, (see MANAGEMENT OF ADVERSE REACTIONS section.)
DOSAGE AND ADMINISTRATION
A 0.4% or 0.8% solution of Lidocaine Hydrochloride and 5% Dextrose Injection, USP is not suitable for bolus administration and is to be used only for infusion following appropriate bolus administration.
IN THE ADULT PATIENT, DOSAGE SHOULD BE LIMITED TO NO MORE THAN 200 TO 300 MG OF LIDOCAINE ADMINISTERED DURING A ONE-HOUR PERIOD.
Patients with reduced hepatic function or diminished hepatic blood flow (as in heart failure and after cardiac surgery), or those over 70 years of age, should receive half the usual loading dose and also should be given lower maintenance levels of intravenous lidocaine. Patients over 65 years may benefit from dosing based upon body weight.
For continuous intravenous infusion, in patients whose arrhythmia tends to recur following a temporary response to a single or once repeated direct injection and who are incapable of receiving oral antiarrhythmic therapy, lidocaine hydrochloride may be infused continuously in a concentration of 0.4% (4 mg/mL) or 0.8% (8 mg/mL) at a rate of 1 to 4 mg (0.25 to 1 mL of 0.4% or 0.125 to 0.5 mL of 0.8%) per minute (20 to 50 micrograms/kg/minute) in the average 70 kg adult. I.V. infusion of the drug must be administered under constant ECG monitoring to avoid potential overdosage and toxicity. I.V. infusion should be terminated as soon as the patient’s basic cardiac rhythm appears to be stable or at the earliest signs of toxicity. As soon as possible, and when indicated, patients should be changed to an oral antiarrhythmic agent for maintenance therapy.
Prolonged (24 hour) infusion of the drug also appears to result in a longer half-life and reduced rate of clearance (even in patients without cardiac or hepatic failure) that may result in toxic accumulation of lidocaine in the plasma. After the first 24 hours, the rate of infusion of lidocaine should be reduced by about one-half to compensate for the drop in the rate of elimination of the drug.
When administering lidocaine hydrochloride (or any potent medication) by continuous intravenous infusion, it is advisable to use a precision volume control I.V. set.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. (See PRECAUTIONS.)
INSTRUCTIONS FOR USE
To Open:
Tear outer wrap at notch and remove solution container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
Preparation for Administration
(Use aseptic technique)
-
Close flow control clamp of administration set.
-
Remove cover from outlet port at bottom of container.
-
Insert piercing pin of administration set into port with a twisting motion until the set is firmly seated. NOTE: See full directions on administration set carton.
-
Suspend container from hanger.
-
Squeeze and release drip chamber to establish proper fluid level in chamber.
-
Open flow control clamp and clear air from set. Close clamp.
-
Attach set to venipuncture device. If device is not indwelling, prime and make venipuncture.
-
Regulate rate of administration with flow control clamp.
WARNING: Do not use flexible container in series connections.
HOW SUPPLIED
0.4% Lidocaine Hydrochloride and 5% Dextrose Injection, USP and 0.8% Lidocaine Hydrochloride and 5% Dextrose Injection, USP are supplied in single-dose flexible containers.
| NDC No. | Product | Container Size (mL) |
| 0409–7931–32 | 0.4% Lidocaine Hydrochloride and 5% Dextrose Injection, USP | 250 |
| 0409–7931–24 | 0.4% Lidocaine Hydrochloride and 5% Dextrose Injection, USP | 500 |
| 0409–7939–32 | 0.8% Lidocaine Hydrochloride and 5% Dextrose Injection, USP | 250 |
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.] Protect from freezing.
Revised: May, 2009
Printed in USA
EN-2140
Hospira, Inc., Lake Forest, IL 60045 USA
IM-0651

IM-0653

| LIDOCAINE HYDROCHLORIDE AND DEXTROSE
lidocaine hydrochloride injection, solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA018388 | 01/26/2010 | |
| LIDOCAINE HYDROCHLORIDE AND DEXTROSE
lidocaine hydrochloride injection, solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA018388 | 01/26/2010 | |
| Labeler - Hospira, Inc. (141588017) |