DIGOXIN
-
digoxin solution
Roxane Laboratories, Inc
----------
DIGOXIN Oral Solution, USP50 mcg (0.05 mg) per mL
Rx only
DESCRIPTION
Digoxin is one of the cardiac glycosides, a closely-related group of plant-derived drugs with shared pharmacological effects. The term "digitalis" is used to designate the whole group. Digoxin is extracted from the leaves of the common foxglove, Digitalis lanata. Like each of the other cardiac glycosides, digoxin consists of a polycyclic core and a sugar side chain. Digoxin’s chemical name is 3β-[0-2,6-dideoxy-β-D-ribo-hexopyranosyl-(1→4)-0-2, 6-dideoxy-β-D-ribo-hexopyranosyl-(1→4)-2, 6-dideoxy-β-D-ribo-hexopyranosyl)oxy]-12β, 14-dihydroxy-5β-card-20(22)-enolide; its structural formula is:
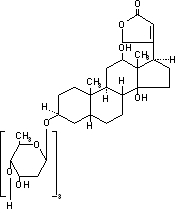
Its molecular formula is C41H64O14, and its molecular weight is 780.95. Digoxin is practically insoluble in water and in ether, slightly soluble in 50% ethanol and in chloroform, and freely soluble in pyridine. Digoxin powder consists of odorless white crystals.
Digoxin Oral Solution, USP is formulated for oral administration, and each mL contains 50 mcg (0.05 mg digoxin). The lime-flavored solution contains the following inactive ingredients: alcohol 10% (by volume at 60°F), glycerin, lime (imitation), methylparaben 0.1%, propylparaben 0.02%, purified water, sodium citrate, and sorbitol solution.
CLINICAL PHARMACOLOGY
Mechanism of Action
All of digoxin’s actions are mediated through its effects on NaK–ATPase. This enzyme, the “sodium pump,” is responsible for maintaining the intracellular milieu throughout the body by moving sodium ions out of and potassium ions into cells. By inhibiting NaK–ATPase, digoxin
- causes increased availability of intracellular calcium in the myocardium and conduction system, with consequent increased inotropy, increased automaticity, and reduced conduction velocity;
- indirectly causes parasympathetic stimulation of the autonomic nervous system, with consequent effects on the sino-atrial (SA) and atrioventricular (AV) nodes;
- reduces catecholamine reuptake at nerve terminals, rendering blood vessels more sensitive to endogenous or exogenous catecholamines;
- increases baroreceptor sensitization, with consequent increased carotid sinus nerve activity and enhanced sympathetic withdrawal for any given increment in mean arterial pressure;
- increases (at higher concentrations) sympathetic outflow from the central nervous system (CNS) to both cardiac and peripheral sympathetic nerves; and
- allows (at higher concentrations) progressive efflux of intracellular potassium, with consequent increase in serum potassium levels.
The cardiologic consequences of these direct and indirect effects are an increase in the force and velocity of myocardial systolic contraction (positive inotropic action), a slowing of the heart rate (negative chronotropic effect), and decreased conduction velocity through the AV node.
Pharmacokinetics
Absorption
Following oral administration, peak serum concentrations of digoxin occur at 30 to 90 minutes. In children and in adult volunteers, absolute bioavailability of digoxin from the solution formulation is 70 to 85%, similar to that seen (in adults) with standard tablets (60 to 80%). When the solution is taken after meals, the peak serum concentrations increase by 20% and the total amount of digoxin absorbed increases by 43%, but the rate of digoxin absorption is unchanged. When taken with meals high in bran fiber, however, the amount absorbed from an oral dose may be reduced. Digoxin absorption may also be affected by various concomitant therapy modulating gastric pH and P-glycoprotein; see PRECAUTIONS: Drug Interactions.
Comparisons of the systemic availability and equivalent doses for preparations of digoxin are shown in Table 1: Comparisons of the Systemic Availability and Equivalent Doses for Preparations of Digoxin.
Table 1: Comparisons of the Systemic Availability and Equivalent Doses for Preparations of Digoxin
|
|||||
|
Product | Absolute Bioavailability |
Equivalent Doses (mcg)* Among Dosage Forms |
|||
| Tablets | 60-80% | 62.5 | 125 | 250 | 500 |
| Solution | 70-85% | 62.5 | 125 | 250 | 500 |
| Capsules | 90-100% | 50 | 100 | 200 | 400 |
| Injection/IV | 100% | 50 | 100 | 200 | 400 |
In some patients, orally administered digoxin is converted to inactive reduction products (e.g., dihydrodigoxin) by colonic bacteria in the gut. Data suggested that one in ten patients treated with digoxin will degrade 40% or more of the ingested dose. As a result, certain antibiotics may increase the absorption of digoxin in such patients. The magnitude of rise in serum digoxin concentration relates to the extent of bacterial inactivation, and may be as much as two-fold in some cases.
Distribution
Following drug administration, a 6- to 8-hour tissue distribution phase is observed. This is followed by a much more gradual decline in the serum concentration of the drug, which is dependent on the elimination of digoxin from the body. Clinical evidence indicates that the early high serum concentrations do not reflect the concentration of digoxin at its sites of action, but that with chronic use, the steady-state post-distribution serum concentrations are in equilibrium with tissue concentrations. In individual patients, these post-distribution serum concentrations may be useful in evaluating therapeutic and toxic effects.
Digoxin is concentrated in tissues and therefore has a large apparent volume of distribution. Digoxin crosses both the blood-brain barrier and the placenta. At delivery, the serum digoxin concentration in the newborn is similar to the serum concentration in the mother. Approximately 25% of digoxin in the plasma is bound to protein. Serum digoxin concentrations are not significantly altered by large changes in fat tissue weight, so that its distribution space correlates with lean (i.e., ideal) body weight, not total body weight.
Metabolism
Only a small percentage of digoxin is metabolized. The end metabolites, which include 3-β-digoxigenin, 3-keto-digoxigenin, and their glucuronide and sulfate conjugates, are polar in nature and are postulated to form via hydrolysis, oxidation, and conjugation. The metabolism of digoxin is not dependent on cytochrome P-450 system, and digoxin is not known to induce or inhibit the cytochrome P-450 system.
Excretion
Elimination of digoxin is predominantly renal, although in adult volunteers about a quarter of serum digoxin is eliminated through the intestinal lumen, excreted in bile or secreted directly into the lumen by P-glycoprotein.
The serum half-life of digoxin in children is reported to be 18 to 36 hours, and in adults it is typically 36 to 48 hours. The half-life in anuric patients is prolonged to 3.5 to 5 days.
Digoxin is not effectively removed from the body by dialysis, exchange transfusion, or cardiopulmonary bypass because most of the drug is bound to tissue.
Special Populations
Geriatrics
The dose of digoxin in geriatric population should be adjusted based on renal function.
Gender
No clinically significant gender differences in digoxin pharmacokinetics have been reported.
Race
Racial differences in digoxin pharmacokinetics have not been formally studied.
Renal Insufficiency
The clearance of digoxin can be primarily correlated with renal function as indicated by creatinine clearance. Table 3: Usual Daily Maintenance Dose Requirements (mcg) of Digoxin for Estimated Peak Body Stores of 10 mcg/kg in Adults provides the usual daily maintenance dose requirements of solution based on creatinine clearance (per 70 kg) for adults (see DOSAGE AND ADMINISTRATION).
For children with known or suspected renal dysfunction, lower starting doses should be considered combined with frequent monitoring of digoxin levels
Hepatic Insufficiency
Plasma digoxin concentrations in patients with acute hepatitis generally fall within the range of profiles in a group of healthy subjects.
Thyroid Status
In hyperthyroidism lower serum digoxin concentrations have been reported due to decreased absorption of digoxin. Hypothyroid patients may require smaller doses of digoxin due to higher serum digoxin concentrations observed in such patients.
Malabsorption Conditions
The absorption of digoxin is reduced in some malabsorption conditions.
Assays
Assays of digoxin levels in serum are widely available. Digoxin levels in saliva do not correlate well with those in serum.
Endogenous substances of unknown composition (digoxin-like immunoreactive substances, DLIS) can interfere with standard radioimmunoassays for digoxin. The interference most often causes results to be falsely positive or falsely elevated, but sometimes it causes results to be falsely reduced. Some assays are more subject to these failings than others. An LC/MS/MS method is available that may provide a more accurate determination of plasma digoxin levels, however, clinical trials have not been conducted to determine its susceptibility to DLIS interference. DLIS are present in up to half of all neonates and in varying percentages of pregnant women, patients with hypertrophic cardiomyopathy, patients with renal or hepatic dysfunction, and other patients who are volume-expanded for any reason. The measured levels of DLIS (as digoxin equivalents) are usually low (0.2 to 0.4 ng/mL), but sometimes they reach levels that would be considered therapeutic or even toxic.
In some assays spironolactone may be falsely detected as digoxin, at levels up to 0.5 ng/mL. Some traditional Chinese medicines cause similar interference.
Spironolactone and DLIS are much more extensively protein-bound than digoxin. As a result, assays of free digoxin levels (which tend to be about 25% less than total levels, consistent with the usual extent of protein binding) are not affected by spironolactone or DLIS.
Clinical Trials
Chronic Heart Failure
Two small 12-week, double-blind, randomized trials compared digoxin to placebo in adult patients with chronic congestive heart failure, New York Heart Association Class II or III. The enrolled patients had all been receiving digoxin before the trials, but this was withdrawn before randomization. They continued to receive diuretics and (in the larger trial) ACE inhibitors. The trials enrolled 178 and 88 patients, respectively. In each of these trials, randomization to digoxin was associated with better preservation of exercise capacity and with reduced need of failure-related hospitalization, emergency care, and concomitant heart-failure therapy. NYHA class and patients' global assessments were also improved, although this effect achieved statistical significance only in the larger of the two studies.
The Digitalis Investigation Group (DIG) main trial was a 37-week, multicenter, randomized, double-blind mortality study comparing digoxin to placebo in 6800 adult patients with heart failure and left ventricular ejection fraction ≤0.45. At randomization, 67% were NYHA class I or II, 71% had heart failure of ischemic etiology, 44% had been receiving digoxin, and most were receiving a concomitant ACE inhibitor (94%) and diuretics (82%). As in the smaller trials described above, patients who had been receiving open-label digoxin were withdrawn from this treatment before randomization. Randomization to digoxin was again associated with a significant reduction in the incidence of hospitalization, whether scored as number of hospitalizations for heart failure (relative risk 75%), risk of having at least one such hospitalization during the trial (RR 72%), or number of hospitalizations for any cause (RR 94%). On the other hand, randomization to digoxin had no apparent effect on mortality (RR 99%, with confidence limits of 91 to 107%).
Atrial Fibrillation
In 3 different randomized, double-blind trials that included a total of 315 adult patients, digoxin was compared to placebo for the conversion of recent-onset atrial fibrillation to sinus rhythm. Conversion was equally likely, and equally rapid, in the digoxin and placebo groups. In a randomized 120-patient trial comparing digoxin, sotalol, and amiodarone, patients randomized to digoxin had the lowest incidence of conversion to sinus rhythm, and the least satisfactory rate control when conversion did not occur.
Digoxin has also been studied as a means of controlling the ventricular response to chronic atrial fibrillation in adults. Digoxin was effective at reducing the resting heart rate, but not at reducing the heart rate during exercise.
In at least one study, digoxin was studied as a means of delaying reversion to atrial fibrillation in adult patients with frequent recurrence of this arrhythmia. This was a randomized, double-blind, 43-patient crossover study. Digoxin increased the mean time between symptomatic recurrent episodes by 54%, but had no effect on the frequency of fibrillatory episodes seen during continuous electrocardiographic monitoring.
No controlled randomized study of digoxin in children with atrial tachyarrhythmias has been done.
Atrial Flutter
There are no reports of controlled trials of digoxin for conversion of atrial flutter, rate control during atrial flutter, or reduction of the frequency of recurrence of atrial flutter in adults.
Supraventricular Tachycardia
There are no reports of controlled trials of digoxin for conversion of supraventricular tachycardia (SVT), rate control during SVT, or reduction of the frequency of recurrence of SVT in adults.
INDICATIONS AND USAGE
Heart Failure
Digoxin Oral Solution, USP is indicated for the treatment of mild to moderate heart failure. Digoxin increases left ventricular ejection fraction and improves heart failure symptoms as evidenced by exercise capacity and heart failure-related hospitalizations and emergency care, while having no effect on mortality. Where possible, digoxin should be used with a diuretic and an angiotensin-converting enzyme inhibitor, but an optimal order for starting these three drugs cannot be specified.
Digoxin increases myocardial contractility in children with congestive heart failure.
Atrial Fibrillation
Digoxin Oral Solution, USP is indicated for the control of resting ventricular response rate in patients with chronic atrial fibrillation.
There are no controlled randomized studies of digoxin in children with atrial tachyarrhythmias.
CONTRAINDICATIONS
Allergy to digoxin is rare. In such patients (who may or may not have similar reactions to other forms of digitalis), the use of digoxin is contraindicated. Digitalis glycosides are contraindicated in ventricular fibrillation.
WARNINGS
Patients with Wolff-Parkinson-White syndrome who develop atrial fibrillation are at high risk of ventricular fibrillation. Treatment of these patients with digoxin leads to greater slowing of conduction in the atrioventricular node than in accessory pathways, and the risks of rapid ventricular response leading to ventricular fibrillation are thereby increased.
By slowing conduction in nodal tissue, digoxin administration can exacerbate Sick Sinus Syndrome and can cause a greater degree of atrioventricular block.
Some signs and symptoms (anorexia, nausea, vomiting, and certain arrhythmias) can equally result from digoxin toxicity as from congestive heart failure. Misidentification of their etiology might lead the clinician to continue or increase digoxin dosing, when dosing should actually be suspended. When the etiology of these signs and symptoms is not obvious, measurement of serum digoxin levels may be helpful.
In patients with hypertrophic cardiomyopathy (formerly called idiopathic hypertrophic subaortic stenosis), the positive inotropic effect of digoxin leads to an increased subvalvular outflow gradient. Digoxin is rarely beneficial in patients with this condition.
Chronic constrictive pericarditis is not generally associated with any inotropic defect, so heart failure of this etiology is unlikely to respond to treatment with digoxin. By slowing the resting heart rate, digoxin may actually decrease cardiac output in these patients.
Patients with amyloid heart disease may be more susceptible to toxicity from digoxin.
Digoxin is of limited value in patients with restrictive cardiomyopathies, although it has been used for rate control in the subgroup of patients with atrial fibrillation.
PRECAUTIONS
General
Even at serum levels of digoxin within the conventional therapeutic range (0.5 to 2 ng/mL), the risk of digoxin toxicity is increased by hypokalemia. Serum potassium levels should be carefully monitored when digoxin is given to patients at high risk of hypokalemia (e.g., those receiving diuretics, corticosteroids, or other drugs that commonly lead to potassium loss; those with gastrointestinal losses through diarrhea, vomiting, or nasogastric suction; or those with potassium-losing endocrinopathies or nephropathies).
Digoxin toxicity is also more likely in the presence of hypomagnesemia. Hypomagnesemia is common in most of the same conditions in which hypokalemia appears. It is also commonly seen in alcoholics and in patients with diabetes mellitus or hypercalcemia.
Because digoxin’s therapeutic and toxic effects are all largely mediated by intracellular calcium distribution, they are affected by abnormalities in serum calcium levels. Hypercalcemia increases the risk of digoxin toxicity, while digoxin may be therapeutically ineffective in the presence of hypocalcemia.
Reduction of digoxin dosage may be desirable prior to electrical cardioversion to avoid induction of ventricular arrhythmias, but the physician must consider the consequences of a rapid increase in ventricular response to atrial fibrillation if digoxin is withheld 1 to 2 days prior to cardioversion. If there is a suspicion that digitalis toxicity exists, elective cardioversion should be delayed. If it is not prudent to delay cardioversion, the energy level selected should be minimal at first and carefully increased in an attempt to avoid precipitating ventricular arrhythmias.
Atrial arrhythmias associated with hypermetabolic states (e.g., hyperthyroidism) are particularly resistant to digoxin treatment. Large doses of digoxin are not recommended as the only treatment of these arrhythmias and care must be taken to avoid toxicity if large doses of digoxin are required. In hypothyroidism, the digoxin requirements are reduced. Digoxin responses are normal in patients with compensated thyroid disease.
Laboratory Tests
At normal heart rates, therapeutic levels of digoxin are associated with characteristic “scooping” changes in the S–T segment of the electrocardiogram; these changes are unlikely to be misinterpreted. During exercise, however, the same serum levels of digoxin can lead to changes that may be indistinguishable from those of ischemia.
Assays of serum digoxin levels are described in CLINICAL PHARMACOLOGY, and their use in patient monitoring is described in DOSAGE AND ADMINISTRATION.
Drug Interactions
Drugs that induce/inhibit P-glycoprotein in intestine or kidney have the potential to alter digoxin pharmacokinetics.
Cardiovascular Drugs
Antiarrhythmics
In patients receiving digoxin therapy, significant increases in serum digoxin concentrations have been reported with amiodarone, propafenone and quinidine. On initiation of these medications, the need for digitalis therapy should be reviewed and the dose reduced by approximately 50% or discontinued. If digitalis treatment is continued, serum levels should be closely monitored and patients observed for clinical evidence of toxicity.
No significant changes in digoxin pharmacokinetics have been reported with disopyramide, dofetilide, flecainide, moricizine, mexilitine, procainamide, or sotalol.
Calcium Channel Blockers
Verapamil
Clinical use of verapamil in digitalized patients has shown the combination to be well tolerated if digoxin doses are properly adjusted. However, chronic verapamil treatment can increase serum digoxin levels by 50% to 75% during the first week of therapy, and this can result in digitalis toxicity. In patients with hepatic cirrhosis the influence of verapamil on digoxin kinetics is magnified. Verapamil may reduce total body clearance and extrarenal clearance of digitoxin by 27% and 29%, respectively. Maintenance and digitalization doses should be reduced when verapamil is administered, and the patient should be reassessed to avoid over- to- under-digitalization. Whenever over-digitalization is suspected, the daily dose of digitalis should be reduced or temporarily discontinued. On discontinuation of verapamil use, the patient should be reassessed to avoid under-digitalization. In previous clinical trials with other verapamil formulations related to the control of ventricular response in digitalized patients who had atrial fibrillation or atrial flutter, ventricular rates below 50/min at rest occurred in 15% of patients, and asymptomatic hypotension occurred in 5% of patients.
Digoxin levels should be monitored when initiating, adjusting, and discontinuing diltiazem, nifedipine, nitrendipine therapy to avoid possible over- or under-digitalization.
No significant changes in digoxin pharmacokinetics have been reported with amlodipine, felodipine, isradipine, nicardipine, or nisoldipine.
ACE Inhibitors
Captopril
In a study of young healthy male subjects no evidence of a direct pharmacokinetic captopril digoxin interaction could be found. Concomitant administration of captopril with digoxin increases serum digoxin concentration (Cmax) by 58% and exposure (AUC) by 39% in patients with severe (NYHA Class IV) congestive heart failure. No patient developed evidence of digoxin toxicity.
No significant changes in digoxin pharmacokinetics have been reported with benazepril, enalapril, lisinopril, moexipril, perindopril, quinapril, ramipril, or trandolapril.
Angotensin-II Blockers
Telmisartan
When telmisartan was coadministered with digoxin, increases in median digoxin peak (49%) and in trough plasma concentration (20%) were observed. It is therefore recommended that digoxin levels be monitored when initiating, adjusting, and discontinuing telmisartan to avoid possible over- or under-digitalization.
No significant changes in digoxin pharmacokinetics have been reported with candesartan, eprosartan, irbesartan, losartan, olmesartan, or valsartan.
Diuretics
Spironolactone
Spironolactone increases the plasma concentrations of digoxin by reducing its renal clearance.
No signficant changes in digoxin pharmacokinetics have been reported with torsemide or triamterene.
Beta Blockers
Carvedilol
Following concomitant administration of carvedilol (25 mg once daily) and digoxin (0.25 mg once daily) for 14 days, steady-state AUC and trough concentrations of digoxin were increased by 14% and 16%, respectively, in 12 hypertensive patients. In eight children (age 2 weeks to 8 years), the oral clearance of digoxin decreased by 50% with carvedilol.
No signficant changes in digoxin pharmacokinetics have been reported with bisoprolol or esmolol.
Other Cardiovascular Drugs
Epoprostenol
In a pharmacokinetic substudy in patients with congestive heart failure receiving digoxin in whom therapy with epoprostenol was initiated, apparent oral clearance values for digoxin were decreased by 15% on the second day of therapy and had returned to baseline value by day 87. Patients on digoxin may show elevations of digoxin concentrations after initiation of therapy with epoprostenol, which may be clinically significant in patients prone to digoxin toxicity.
No significant changes in digoxin pharmacokinetics have been reported with aspirin, bosentan, nesiritide, or prazosin.
Antithrombotic Drugs
No significant changes in digoxin pharmacokinetics have been reported with argatroban and fondaparinux.
Platelet Aggregation Inhibitors
No significant changes in digoxin pharmacokinetics have been reported with clopidogrel, dipyridamole, or ticlopidine.
Lipid Lowering Drugs
No significant changes in digoxin pharmacokinetics have been reported with atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, simvastatin, colesevelam, or ezetimibe.
Noncardiovascular Drugs
CNS Drugs
Alprazolam
The therapeutic doses of alprazolam do not significantly alter digoxin pharmacokinetics in healthy subjects. However, in older patients significant increases in digoxin exposure have been reported.
No significant changes in digoxin pharmacokinetics have been reported with citalopram, donepezil, escitalopram, galantamine, levetiracetam, paroxetine, rivastigmine, ropinirole, sertraline, tiagabine, topiramate, zaleplon, or zolpidem.
Nonsteroidal Antiinflammatory Drugs
Diclofenac and indomethacin have been reported to elevate digoxin levels. Patients should be monitored for possible digoxin toxicity.
No significant changes in digoxin pharmacokinetics have been reported with rofecoxib.
Antifungal Drugs
Ketoconazole and itraconazole have been reported to elevate plasma concentrations of digoxin.
No significant changes in digoxin pharmacokinetics have been reported with terbinafine or voriconazole.
Antisecretory Drugs
Rabeprazole
In normal subjects, co-administration of rabeprazole 20 mg QD resulted in an increase in the AUC and Cmax for digoxin of 19% and 29%, respectively. Patients may need to be monitored when digoxin is taken concomitantly with rabeprazole.
Lansoprazole, esomeprazole, and omeprazole inhibit gastric acid secretion and may interfere with the absorption of digoxin.
No significant changes in digoxin pharmacokinetics have been reported with pantoprazole.
Antibacterial Drugs
Gatifloxacin
Few cases of elevated serum concentrations of digoxin have been reported in patients receiving digoxin and gatifloxacin. Although dose adjustments for digoxin are not warranted with initiation of gatifloxacin treatment, patients taking digoxin should be monitored for signs and symptoms of toxicity. In patients who display signs and symptoms of digoxin intoxication, serum digoxin concentrations should be determined, and digoxin dosage should be adjusted as appropriate.
Clarithromycin
Elevated digoxin concentrations have been reported in patients receiving clarithromycin and digoxin concomitantly due to inhibition of intestinal and renal P-glycoprotein. Some patients have shown clinical signs consistent with digoxin toxicity, including potentially fatal arrhythmias. Serum digoxin levels should be carefully monitored while patients are receiving digoxin and clarithromycin simultaneously.
Azithromycin, erythromycin and tetracycline have also been reported to elevate digoxin levels.
No significant changes in digoxin pharmacokinetics have been reported with gemifloxacin, levofloxacin, moxifloxacin, or trovafloxacin.
Antitubercular Drugs
Rifampin
Rifampin may decrease serum digoxin concentration, especially in patients with renal dysfunction, by increasing the non-renal clearance of digoxin. Rifampin reduced the oral bioavailability of digoxin from 63% to 44% in healthy volunteers mainly by inducing P-glycoprotein in the small intestine.
Antiviral Drugs
Foscarnet
Physical incompatibility has been reported with digoxin. Digoxin should not be administered concurrently via the same catheter.
No significant changes in digoxin pharmacokinetics have been reported with famciclovir or valacyclovir.
Glucose Lowering Drugs
Acarbose
Acarbose has been shown to decrease the bioavailability of digoxin when they are co-administered, which may require digoxin dose adjustment.
Miglitol
In healthy volunteers, co-administration of 50 mg or 100 mg miglitol and digoxin reduced the average plasma concentrations of digoxin by 19% and 28%, respectively. However, in diabetic patients under treatment with digoxin, plasma digoxin concentrations were not altered by co-administration of miglitol 100 mg for 14 days.
No significant changes in digoxin pharmacokinetics have been reported with nateglinide, repaglinide, pioglitazone, or rosiglitazone.
Immunosuppressive Drugs
Cylcosporine
Reduced clearance and apparent volume of distribution of digoxin has been observed when coadministered with cyclosporine. Severe digitalis toxicity has been seen within days of starting cyclosporine.
No significant changes in digoxin pharmacokinetics have been reported with sirolimus.
Drugs for Benign Prostatic Hyperplasia
No significant changes in digoxin pharmacokinetics have been reported with alfuzosin, dutasteride, finasteride, or tamsulosin.
Drugs for Respiratory Disorders
Albuterol and salbutamol have been reported to lower serum digoxin levels. Nevertheless, it would be prudent to evaluate carefully the serum digoxin levels.
No significant changes in digoxin pharmacokinetics have been reported with montelukast.
Drugs Interfering with Absorption
Propantheline and diphenoxylate, by decreasing gut motility, may increase digoxin absorption. Activated charcoal, antacids, kaolin-pectin, sulfasalazine, neomycin, cholestyramine, certain anticancer drugs, metoclopramide, meals high in bran, sucralfate may interfere with digoxin absorption, resulting in unexpectedly low serum concentrations.
Colestipol Hydrochloride
Colestipol can bind digoxin. Discontinuing colestipol hydrochloride could pose a hazard to health if a potentially toxic drug that is significantly bound to the resin has been titrated to a maintenance level while the patient was taking colestipol hydrochloride.
Dietary Products
Grapefruit Juice
Grapefruit juice had no significant effect on the maximum plasma drug concentration (Cmax) of digoxin (0.5 mg) or the overall exposure. The digoxin renal clearance remained unchanged.
Herbal Products
St. John’s Wort
Administration of St. John’s Wort extract to 8 healthy male volunteers during 14 days resulted in a 18% decrease in exposure (AUC) after a single digoxin dose (0.5 mg) due to 1.4-fold increase in duodenal P-glycoprotein activity.
Other Digoxin-Drug Interaction Information
Post-marketing surveillance of tramadol has revealed rare reports of digoxin toxicity. Doxazosin, ketorolac, meloxicam, and mycophenolate have no effect on protein binding of digoxin. In vitro studies indicated that ertapenem had no effect on P-glycoprotein transport of digoxin. As a cationic drug that is eliminated by renal tubular secretion, digoxin has the potential for interaction with metformin by competing for common renal tubular transport systems.
There have been inconsistent reports regarding the effects of other drugs (e.g., penicillamine, quinine) on serum digoxin concentration.
No significant changes in digoxin pharmacokinetics have been reported with acitretin, aprepitant, orlistat, raloxifene, sevelamer, or tegaserod.
Pharmacodynamic Interactions
Dofetilide
In patients, the concomitant administration of digoxin with dofetilide was associated with a higher rate of Torsade de pointes.
Moricizine
Moricizine has been reported to increase the PR interval and QRS duration. There are reports of first-degree atrioventricular block or bundle branch block developing in patients with concomitant digitalis administration. The known effects of moricizine on calcium conductance may explain the effects on atrioventricular node conduction.
Sotalol
Proarrhythmic events were more common in sotalol treated patients also receiving digoxin; it is not clear whether this represents an interaction or is related to the presence of CHF, a known risk factor for proarrhythmia, in the patients receiving digoxin.
Teriparatide
Sporadic case reports have suggested that hypercalcemia may predispose patients to digitalis toxicity. Because teriparatide transiently increases serum calcium, teriparatide should be used with caution in patients taking digitalis.
Potassium-depleting corticosteroids and diuretics may be major contributing factors to digitalis toxicity.
Calcium, particularly if administered rapidly by the intravenous route, may produce serious arrhythmias in digitalized patients.
Treatment of hypothyroidism in patients taking digoxin may increase the dose requirements of digoxin. Thyroid administration to a digitalized, hypothyroid patient may increase the dose requirement of digoxin.
Concomitant use of digoxin and sympathomimetics increases the risk of cardiac arrhythmias.
Succinylcholine may cause a sudden extrusion of potassium from muscle cells, and may thereby cause arrhythmias in digitalized patients.
Although β-adrenergic blockers or calcium-channel blockers and digoxin may be useful in combination to control atrial fibrillation, their additive effects on AV node conduction can result in complete heart block.
Due to considerable variability of these interactions, the dosage of digoxin should be individualized when patients receive these medications concurrently. Furthermore, caution should be exercised when combining digoxin with any drug that may cause significant deterioration in renal function, since a decline in glomerular filtration or tubular secretion may impair the excretion of digoxin.
Carcinogenesis, Mutagenesis, Impairment of Fertility
There have been no long-term studies performed in animals to evaluate carcinogenic potential.
Pregnancy
Teratogenic Effects
Pregnancy Category C.
Animal reproduction studies have not been conducted with digoxin. It is also not known whether digoxin can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Digoxin should be given to a pregnant woman only if clearly needed.
Nursing Mothers
Digoxin levels in human milk are lower than those in maternal serum. A human infant ingesting plausible quantities of such milk will receive much less than therapeutic doses of digoxin, even when the mother’s levels are well into the toxic range.
Geriatric Use
Clinical studies of digoxin did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Pediatric Use
Digoxin increases myocardial contractility in children with congestive heart failure. There are no controlled randomized studies of digoxin in children with atrial tachyarrhythmias. See: CLINICAL PHARMACOLOGY: Clinical Trials, INDICATIONS AND USAGE and DOSAGE AND ADMINISTRATION.
ADVERSE REACTIONS
The frequency and severity of adverse reactions to digoxin depend on the dose and route of administration, as well as on the patient's underlying disease or concomitant therapies (see PRECAUTIONS). The overall incidence of adverse reactions has been reported as 5 to 20%, with 15 to 20% of them being considered serious (one to four percent of patients receiving digoxin). Evidence suggests that the incidence of toxicity has decreased since the introduction of the serum digoxin assay and improved standardization of digoxin tablets. Cardiac toxicity accounts for about one-half, gastrointestinal disturbances for about one-fourth, and CNS and other toxicity for about one-fourth of these adverse reactions.
Cardiac
Conduction disturbances or supraventricular tachyarrhythmias, such as atrioventricular (AV) block, atrial tachycardia with or without block and junctional (nodal) tachycardia are the most common arrhythmias associated with digoxin toxicity in children. Ventricular arrhythmias, such as unifocal or multiform ventricular premature contractions, especially in bigeminal or trigeminal patterns, are less common. Ventricular tachycardia may result from digitalis toxicity. Sinus bradycardia may also be a sign of impending digoxin intoxication, especially in infants, even in the absence of first degree heart block. Any arrhythmias or alteration in cardiac conduction that develops in a child taking digoxin should initially be assumed to be a consequence of digoxin intoxication.
Gastrointestinal
Anorexia, nausea, vomiting and diarrhea may be early symptoms of overdosage. However, uncontrolled heart failure may also produce such symptoms.
CNS
Visual disturbances (blurred or yellow vision), headache, weakness, apathy and psychosis can occur. These may be difficult to recognize in infants and children. In one reported case, asymmetric chorea was seen at high digoxin levels, and reappeared when similar levels were inadvertently re-achieved.
Other
Gynecomastia is occasionally observed.
OVERDOSAGE
Digoxin should be discontinued until all signs of toxicity are gone. Discontinuation may be all that is necessary if toxic manifestations are not severe and appear only near the expected time for maximum effect of the drug.
Potassium salts may be used, particularly if hypokalemia is present. Potassium chloride in divided oral doses totaling 1 to 1.5 mEq K+ per kilogram (kg) body weight may be given provided renal function is adequate (1 gram of potassium chloride contains 13.4 mEq K+). When correction of the arrhythmia with potassium is urgent and the serum potassium concentration is low or normal, approximately 0.5 mEq/kg of potassium per hour may be given intravenously in 5% dextrose injection. The intravenous solution of potassium should be dilute enough to avoid local irritation; however, especially in infants care must be taken to avoid intravenous fluid overload. ECG monitoring should be performed to watch for any evidence of potassium toxicity (e.g., peaking of T waves) and to observe the effect on the arrhythmia. The infusion may be stopped when the desired effect is achieved.
Note: Potassium should not be used and may be dangerous in heart block due to digoxin, unless primarily related to supraventricular tachycardia.
Because of its large extravascular volume of distribution, digoxin is not effectively removed from the body by dialysis, by exchange transfusion, or during cardiopulmonary bypass.
Multiple doses of activated charcoal have been found effective in at least 1 case report, and may be of use while the need for and availability of digoxin specific antibody fragments are being assessed. In advanced heart block, temporary ventricular pacing may be beneficial.
Digoxin Immune Fab (Ovine) [DIGIBIND®, DIGIFAB®] may be indicated for the treatment of patients with life-threatening or potentially life-threatening digoxin toxicity or overdose.
DOSAGE AND ADMINISTRATION
Because the pharmacokinetics of digoxin are complex, and because toxic levels of digoxin are only slightly higher than therapeutic levels, digoxin dosing can be difficult. The recommended approach is to
- estimate the patient’s daily maintenance dose;
- adjust the estimate to account for patient-specific factors;
- choose a dosing regimen;
- monitor the patient for toxicity and for therapeutic effect, if possible; and
- adjust the dose.
Table 2: Estimate the Daily Maintenance Dose
The recommended initial estimates for daily oral doses of digoxin are
| Age | Daily Oral Dose, mcg/kg/day |
| Children > 2 years | 10 |
| Prepubertal children | 10 |
| Adults | 3 |
These doses will, in uncomplicated patients, tend to produce steady-state post-absorptive levels of 1 to 2 ng/mL, but the confidence limits around this range are wide.
Adjust the Estimated Dose
As noted in CLINICAL PHARMACOLOGY: Pharmacokinetics and in PRECAUTIONS: Drug Interactions, the body’s handling of digoxin can be affected by many different patient-specific factors. Some of the possible effects are usually small, so anticipatory dose adjustment might not be required, but others should be considered before initial dosing. Patients with abnormal renal function need to have their doses of digoxin proportionately reduced. Normal developmental changes in pediatric renal function were factored into the table given in the previous subsection, but age-related (or other) changes in adult renal function were not. The effects of renal function on recommended digoxin doses in adults is shown in Table 3: Usual Daily Maintenance Dose Requirements (mcg) of Digoxin for Estimated Peak Body Stores of 10 mcg/kg in Adults below. For children with known or suspected renal dysfunction, lower starting doses should be considered combined with frequent monitoring of digoxin levels.
Digoxin’s volume of distribution is proportional to lean body weight, and the table in the previous section assumes that patients are of average composition. The dose of digoxin must be reduced in patients whose lean body weight is — typically because of obesity or edema — an abnormally small fraction of their total body mass.
Concomitant drug use should be considered when adjusting the estimated digoxin dose (see PRECAUTIONS: Drug Interactions).
NOTE: The calibrated dropper supplied with the 60 mL bottle of digoxin oral solution is not appropriate to measure doses below 0.2 mL. Doses less than 0.2 mL require appropriate methods or measuring devices designed to administer an accurate amount to the patient.
Table 3: Usual Daily Maintenance Dose Requirements (mcg) of Digoxin for Estimated Peak Body Stores of 10 mcg/kg in Adults provides average daily maintenance dose requirements of digoxin for patients with heart failure based upon lean body weight and renal function.
Table 3: Usual Daily Maintenance Dose Requirements (mcg) of Digoxin for Estimated Peak Body Stores of 10 mcg/kg in Adults
|
||||||||
|
Corrected Ccr (mL/min/70 kg)* | Lean Body Weight | Number of Days Before Steady State Achieved |
||||||
| kg lb | 50 110 | 60 132 | 70 154 | 80 176 | 90 198 | 100 | ||
| 220 | ||||||||
| 0 | 62.5† | 125 | 125 | 125 | 187.5 | 187.5 | 22 | |
| 10 | 125 | 125 | 125 | 187.5 | 187.5 | 187.5 | 19 | |
| 20 | 125 | 125 | 187.5 | 187.5 | 187.5 | 250 | 16 | |
| 30 | 125 | 187.5 | 187.5 | 187.5 | 250 | 250 | 14 | |
| 40 | 125 | 187.5 | 187.5 | 250 | 250 | 250 | 13 | |
| 50 | 187.5 | 187.5 | 250 | 250 | 250 | 250 | 12 | |
| 60 | 187.5 | 187.5 | 250 | 250 | 250 | 375 | 11 | |
| 70 | 187.5 | 250 | 250 | 250 | 250 | 375 | 10 | |
| 80 | 187.5 | 250 | 250 | 250 | 375 | 375 | 9 | |
| 90 | 187.5 | 250 | 250 | 250 | 375 | 500 | 8 | |
| 100 | 250 | 250 | 250 | 375 | 375 | 500 | 7 | |
Table 4: Daily Dose in Milliliters
| Target Dose in mcg/kg/day: | ||||||||
| 2 | 3 | 4 | 5 | 6 | 8 | 10 | ||
|
WeightIn kg | 10 | 0.4 | 0.6 | 0.8 | 1 | 1.2 | 1.6 | 2 |
| 11 | 0.44 | 0.66 | 0.88 | 1.1 | 1.32 | 1.76 | 2.2 | |
| 12 | 0.48 | 0.72 | 0.96 | 1.2 | 1.44 | 1.92 | 2.4 | |
| 13 | 0.52 | 0.78 | 1.04 | 1.3 | 1.56 | 2.08 | 2.6 | |
| 14 | 0.56 | 0.84 | 1.12 | 1.4 | 1.68 | 2.24 | 2.8 | |
| 15 | 0.6 | 0.9 | 1.2 | 1.5 | 1.8 | 2.4 | 3 | |
| 20 | 0.8 | 1.2 | 1.6 | 2 | 2.4 | 3.2 | 4 | |
| 30 | 1.2 | 1.8 | 2.4 | 3 | 3.6 | 4.8 | 6 | |
| 40 | 1.6 | 2.4 | 3.2 | 4 | 4.8 | 6.4 | 8 | |
| 50 | 2 | 3 | 4 | 5 | 6 | 8 | 10 | |
| 60 | 2.4 | 3.6 | 4.8 | 6 | 7.2 | 9.6 | 12 | |
| 70 | 2.8 | 4.2 | 5.6 | 7 | 8.4 | 11.2 | 14 | |
| 80 | 3.2 | 4.8 | 6.4 | 8 | 9.6 | 12.8 | 16 | |
| 90 | 3.6 | 5.4 | 7.2 | 9 | 10.8 | 14.4 | 18 | |
| 100 | 4 | 6 | 8 | 10 | 12 | 16 | 20 | |
On the left side of the chart, locate the patient’s weight in kilograms. At the top of the chart, identify which dose in mcg/kg/day will be used for this patient. The block on the chart at which the two rows (weight and target dose) intersect is the milliliter amount that should be given to the patient.
Patient Monitoring
Dosing as described above may need to be increased or decreased, depending upon the patient’s response, so patients must be monitored for signs of efficacy and toxicity.
When the purpose of digoxin therapy is reduction of resting ventricular response to atrial tachyarrhythmia, a therapeutic digoxin effect may be obvious. In other settings, however, the therapeutic effect of digoxin may be difficult to separate from other developments in the course of the underlying disease.
Similarly, digoxin toxicity may be easily identified when there are pathognomonic findings of new atrioventricular block, or of yellow/green discoloration of vision. Other manifestations of digoxin toxicity (e.g., nausea) might have alternative explanations, and symptoms of toxicity may be difficult to evaluate in small children and other inarticulate patients. It will therefore often be necessary to monitor digoxin therapy by means of serum digoxin levels.
Because hypokalemia or hypomagnesia can greatly increase the risk of digoxin toxicity, it is appropriate to monitor these levels whenever digoxin levels are measured. These assays are widely available.
Digoxin should be allowed to distribute into its volume of distribution before measurement, so specimens for these assays should not be collected until 6 to 8 hours after the time of administration. In general, serum levels below 0.5 ng/mL are unlikely to be beneficial, while levels above 2 ng/mL are associated with increased toxicity without increased benefit. Within the 0.5 to 2 ng/mL range, the inotropic effects of digoxin tend to appear at lower concentrations than the electrophysiological effects.
When decision-making is to be guided by serum digoxin levels, the clinician must consider the possibility of reported concentrations that have been falsely elevated by endogenous digoxin-like immunoreactive substances (see CLINICAL PHARMACOLOGY: Assays). If the assay being used is sensitive to these substances, it may be prudent to obtain a baseline measurement before digoxin therapy is started, and correct later values by the reported baseline level.
Dose Adjustment
The monitoring described above may suggest increases or decreases in digoxin doses. Additional monitoring, and in some cases anticipatory dose adjustment, may be indicated around the time of various changes in the patient’s internal milieu, including
- normal development through childhood;
- concomitant drug use should be considered when adjusting the estimated digoxin dose (see PRECAUTIONS: Drug Interactions);
- new co-administration of an antibiotic, especially if the patient had required high doses of digoxin in order to achieve modest serum concentrations, raising the suspicion that a substantial fraction of administered digoxin was being destroyed by colonic bacteria; and
- changes in renal function (see Table 3: Usual Daily Maintenance Dose Requirements (mcg) of Digoxin for Estimated Peak Body Stores of 10 mcg/kg in Adults above).
HOW SUPPLIED
Digoxin Oral Solution, USP [50 mcg (0.05 mg) per mL] is available in 60 mL (3 mg of digoxin) bottles with child-resistant closures. Once open, the bottles are used with graduated droppers provided in the carton. Starting at 0.2 mL, this 1 mL dropper is marked in divisions of 0.1 mL, corresponding to 5 mcg or 0.005 mg of digoxin.
The National Drug Code designations of the package is
NDC 0054-0057-46 60 mL bottle
Storage
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature] and protect from light.
10001143/05Revised November 2009
© RLI, 2009
Package Label - Digoxin Oral Solution, USP [50 mcg (0.05 mg) per mL]
NDC 0054-0057-46 60 mL bottle
Rx Only
Roxane Laboratories, Inc.

| DIGOXIN
digoxin solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA021648 | 08/26/2004 | |
| Labeler - Roxane Laboratories, Inc (058839929) |
| Registrant - Roxane Laboratories, Inc (058839929) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Boehringer Ingelheim Roxane Inc | 128407710 | MANUFACTURE | |