CARISOPRODOL
-
carisoprodol tablet
Sun Pharmaceutical Industries Limited
----------
Carisoprodol TabletsDESCRIPTION
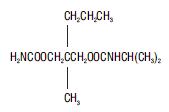
Carisoprodol Tablets, USP is available as 350 mg round, white tablets. Chemically, carisoprodol is N-isopropyl-2-methyl-2-propyl-1,3-propanediol dicarbamate. Carisoprodol is a white, crystalline powder, having a mild, characteristic odor and a bitter taste. It is very slightly soluble in water; freely soluble in alcohol, in chloroform, and in acetone; its solubility is practically independent of pH. Carisoprodol is present as a racemic mixture. The molecular formula is C12H24N2O4, with a molecular weight of 260.33. The structural formula is:
Other Ingredients: lactose, corn starch, microcrystalline cellulose, povidone, talc, croscarmellose sodium, magnesium stearate.
CLINICAL PHARMACOLOGY
Carisoprodol is a centrally acting skeletal muscle relaxant that does not directly relax tense skeletal muscles in man. The mode of action of carisoprodol in relieving acute muscle spasm of local origin has not been clearly identified, but may be related to its sedative properties. In animals, carisoprodol has been shown to produce muscle relaxation by blocking interneuronal activity and depressing transmission of polysynaptic neurons in the spinal cord and in the descending reticular formation of the brain. The onset of action is rapid and lasts four to six hours.
Carisoprodol is metabolized in the liver and is excreted by the kidneys. One of the products of metabolism, meprobamate, is active as an anxiolytic. The degree to which it contributes to the efficacy of carisoprodol is unknown. Carisoprodol is dialyzabic by peritoneal and hemodialysis.
Absorption, Distribution, Metabolism, Excretion
Following a single oral dose of carisoprodol, the time to maximum concentration (Tmax) was 1.98 ± 1.16 hours and the terminal elimination half-life was 2.44 ± 0.93 hours. By normalizing to a single 350 mg carisoprodol tablet, the mean peak concentration (Cmax) was 2.29 ± 0.68 ug/mL, the area under the plasma level time curve (AUC 0-∞) was 10.33 ± 3.87 ug/mL* hour, and the oral clearance (Cl/F) was 39.52 ± 16.83 L/hour. The mean Cmax of metabolite, meprobamate, was 2.08 ± 0.48 ug/mL, a subtherapeutic concentration when compared to a plasma concentration of a single oral meprobamate 400 mg tablet which would yield a meprobamate concentration of approximately 8 ug/mL.
Pharmacokinetics
Absorption
The pharmacokinetics of carisoprodol was determined in a small in vivo biostudy of 5 men and 5 women. When the dose was normalized to 350 mg, the mean peak plasma concentration (Cmax) achieved was 2.29 ± 0.68 ug/mL. Women tended to reach peak plasma concentrations earlier than men (1.45 vs. 2.5 hours) and had a faster apparent oral clearance (0.772 vs. 0.38 L/hour/kg). The clinical significance of these findings is unknown and they may in part be due to the small number of subjects present in the trial.
Metabolism
Carisoprodol is metabolized in the liver via cytochrome P450 enzyme, CYP2C19. This enzyme exhibits genetic polymorphism. For example, 15-20% of Asian populations may be expected to be poor metabolizers. For Caucasians and Blacks, the prevalence of poor metabolizers is 3-5%. Following a single 350 mg dose of carisoprodol, the corresponding normalized peak concentration of meprobamate, which is a metabolite of carisoprodol, was 2.08 ± 0.48 ug/mL. These levels are approximately ¼ of those seen following a single 400 mg dose of meprobamate.
Elimination
Carisoprodol is eliminated by both renal and non-renal routes with a terminal elimination half-life of 2.44 ± 0.93 hours. It is dialyzable by peritoneal and hemodialysis.
Special Populations
The pharmacokinetic profile of carisoprodol in patients with renal impairment or hepatic impairment has not been evaluated. Because carisoprodol is metabolized by the liver and excreted by the kidneys, possible increased exposure of carisoprodol is expected if hepatic and/or renal function is impaired. The drug should be used with caution in patients with impaired hepatic or renal function.
The pharmacokinetic profile of carisoprodol in elderly patients has not been evaluated.
INDICATIONS
Carisoprodol is indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute, painful musculoskeletal conditions.
CONTRAINDICATIONS
Carisoprodol Tablet USP is contraindicated in patients with acute intermittent porphyria, and in patients who are allergic to or who have had idiosyncratic reactions to carisoprodol or meprobamate related compounds.
WARNINGS
Potentially Hazardous Tasks
Patients should be warned that carisoprodol may have sedative properties and may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a motor vehicle or operating machinery.
Drug Dependence, Withdrawal and Abuse
In postmarketing experience, cases of drug abuse, dependence and withdrawal have been reported. Carisoprodol should be used with caution in addiction-prone individuals (See DRUG ABUSE AND DEPENDENCE).
Additive Effects
Since the effects of carisoprodol and alcohol or carisoprodol and other CNS depressants or psychotropic drugs may be additive, appropriate caution should be exercised with patients who take more than one of these agents simultaneously. Concomitant use of carisoprodol and meprobamate is not recommended.
Idiosyncratic Reactions
On very rare occasions, the first dose of carisoprodol has been followed by idiosyncratic symptoms appearing within minutes or hours. Symptoms reported include: extreme weakness, transient quadriplegia, dizziness, ataxia, temporary loss of vision, diplopia, mydriasis, dysarthria, agitation, euphoria, confusion, and disorientation. Symptoms usually subside over the course of the next several hours. Supportive and symptomatic therapy, including hospitalization, may be necessary.
Allergic Reactions
Occasionally, within the period of the first to fourth dose of carisoprodol allergic reactions have occurred in patients who have had no previous contact with the drug. Skin rash, erythema multiforme, pruritus, eosinophilia, and fixed drug eruption have been reported with carisoprodol with cross reaction to meprobamate. Severe reactions have been manifested by asthmatic episodes, fever, weakness, dizziness, angioneurotic edema, smarting eyes, hypotension, and anaphylactoid shock. (See also Idiosyncratic Reactions under "Warnings.")
In case of allergic or idiosyncratic reactions to carisoprodol, discontinue the drug and initiate appropriate symptomatic therapy, which may include epinephrine, antihistamines, and in severe cases corticosteroids.
Usage in Pregnancy and Lactation
The safety of this drug in pregnancy or lactation has not been established. Therefore, use of this drug in pregnancy, in nursing mothers, or in women of childbearing potential requires that the potential benefits of the drug be weighed against the potential hazards to mother and child. Carisoprodol is present in breast milk of lactating mothers at concentrations two to four times that of maternal plasma. This factor should be taken into account when use of the drug is contemplated in breast-feeding patients.
Usage in Children
The efficacy and safety of carisoprodol in patients under 12 years of age has not been determined.
PRECAUTIONS
Seizure Disorders
There have been rare reports of seizures in postmarketing surveillance in temporal association with carisoprodol. These events have involved patients with and without previous medical histories of seizures and have occurred during therapeutic use, with overdose and during withdrawal from prolonged use. The role of carisoprodol in the causality of these seizures has not been established.
Carisoprodol is metabolized in the liver and excreted by the kidney; to avoid its excess accumulation, caution should be exercised in administration to patients with compromised liver or kidney function.
ADVERSE REACTIONS
Cardiovascular– Tachycardia, postural hypotension, and facial flushing.
Central Nervous System– Drowsiness and other CNS effects may require dosage reduction. Also observed: dizziness, vertigo, ataxia, tremor, agitation, irritability, headache, depressive reactions, syncope, seizures, and insomnia. (See also Idiosyncratic Reactions under "Warnings.")
Gastrointestinal– Nausea, vomiting, hiccup, and epigastric distress.
Hematologic– Leukopenia, in which other drugs or viral infection may have been responsible, and pancytopenia, attributed to phenylbutazone, have been reported. No serious blood dyscrasias have been attributed to carisoprodol.
DRUG ABUSE AND DEPENDENCE
In post-marketing experience, drug abuse and dependence have been reported. The majority of cases of drug abuse, dependence and withdrawal have been reported with prolonged use of carisoprodol (beyond several months of treatment) or in combination with other CNS depressant or psychotropic drugs. However, there have been cases reported with carisoprodol alone. Withdrawal symptoms including abdominal cramps, insomnia, headache, nausea, and seizure, have been reported following abrupt cessation after prolonged use. One of the metabolites of carisoprodol, meprobamate, may be habit forming. (See Pharmacokinetics)
Carisoprodol should be used with caution in addiction-prone Individuals, and its use should generally be limited to the acute treatment setting and not for more than 2 – 3 weeks.
OVERDOSAGE
Overdosage of carisoprodol produces CNS depression, and in severe cases coma. Shock, respiratory depression, seizures and death have also been reported rarely. The following signs and symptoms may be associated with carisoprodol overdosage: horizontal and vertical nystagmus, blurred vision, mydriasis, mild tachycardia and hypotension, respiratory depression, euphoria, CNS stimulation, muscular incoordination, and/or rigidity, confusion, headache, hallucinations, and dystonic reactions. The effects of an overdosage of carisoprodol and alcohol or other CNS depressants or psychotropic agents can be additive even when one of the drugs has been taken in the usual recommended dosage. Fatal accidental and non-accidental overdoses have been reported alone or in combination with alcohol or psychotropic drugs.
Treatment of Overdosage
Basic life support measures should be instituted as dictated by the clinical presentation. Induced emesis is not recommended due to the risk of CNS and respiratory depression. Gastric lavage should be considered soon after ingestion (usually within 1 hour). In cases of severe CNS depression, airway protective reflexes may be compromised. In such cases, tracheal intubation should be considered for airway protection and respiratory support. Circulatory support should be administered with volume infusion and pressor agents as indicated. Seizures should be treated with a benzodiazepine IV and may be followed with phenobarbital if seizures recur.
Carisoprodol is metabolized in the liver and excreted by the kidney. The following types of treatment have been used successfully with the related drug meprobamate: activated charcoal (oral or via nasogastric tube), forced diuresis, peritoneal dialysis, and hemodialysis (carisoprodol is dialyzable). Careful monitoring of urinary output is necessary and caution should be taken to avoid overhydration. Observe for possible relapse due to incomplete gastric emptying and delayed absorption. Carisoprodol can be measured in biological fluids by gas chromatography (Douglas, J. F. et al.: J Pharm Sci 58: 145, 1969).
For more information on the management of overdose or unintentional ingestion, contact a Poison Control Center.
DOSAGE AND ADMINISTRATION
The usual adult dosage of carisoprodol tablets, USP is one 350 mg tablet, three times daily and at bedtime. Usage in patients under age 12 is not recommended.
HOW SUPPLIED
Carisoprodol Tablets, USP 350 mg:
Round, convex, white tablets, inscribed with '446' on one side, are available in bottles as follows:
Bottles of 30 (Child Resistant Closure) (NDC 62756-446-01)
Bottles of 100 (Child Resistant Closure) (NDC 62756-446-02)
Bottles of 100 (Non Child Resistant Closure) (NDC 62756-446-03)
Bottles of 500 (Non Child Resistant Closure) (NDC 62756-446-05)
Bottles of 1000 (Non Child Resistant Closure) (NDC 62756-446-04)
Storage: Store at 25°C (77°F); excursions permitted to 15° - 30°C (59° - 86°F) [See USP Controlled Room Temperature]
Dispense in a tight container.
Manufactured by:
Sun Pharmaceutical Industries Ltd.
Acme Plaza, Andheri-Kurla Road,
Andheri (East), Mumbai-400 059, India
Distributed by:
Caraco Pharmaceutical Laboratories, Ltd.
Detroit, MI 48202
ISS. 01/2007
PJPI0107
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - Label - 350 mg
NDC 62756-446-01
Carisoprodol Tablets, USP
350 mg
30 TABLETS
Rx only
SUN PHARMACEUTICAL INDUSTRIES LTD.

| CARISOPRODOL
carisoprodol tablet |
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA040755 | 02/27/2007 | |
| Labeler - Sun Pharmaceutical Industries Limited (650172430) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Sun Pharmaceutical Industries Limited | 725959238 | manufacture, analysis | |