ACEPROMAZINE MALEATE- acepromazine maleate tablet
Boehringer Ingelheim Vetmedica, Inc.
----------
Acepromazine Maleate Tablets
Caution
Federal (U.S.A.) law restricts this drug to use by or on the order of a licensed veterinarian.
Description
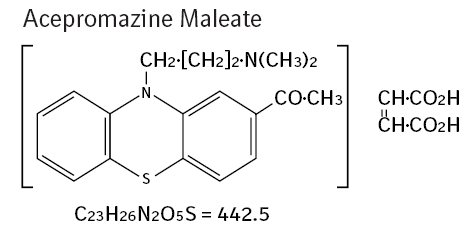
Acepromazine maleate, a potent neuroleptic agent with a low order of toxicity, is of particular value in the tranquilization of dogs. Its rapid action and lack of hypnotic effect are added advantages.
Mode of Action
Acepromazine maleate has a depressant effect on the central nervous system and therefore causes sedation, muscular relaxation and a reduction in spontaneous activity. It acts rapidly, exerting a prompt and pronounced calming effect. It is an effective preanesthetic agent and lowers the dosage requirement of general anesthetics.
Toxicology
Acute and chronic toxicity studies have shown a very low order of toxicity for acepromazine maleate.
A safety study using elevated dosages of Acepromazine Maleate Tablets demonstrated no adverse reactions even when administered at three times the upper limit of the recommended daily dosage (3.0 mg/lb body weight). The clinical observation for this high dosage was mild depression which disappeared in most dogs 24 hours after termination of dosing.
The only occurrence of adverse reaction during numerous clinical trials was a very mild respiratory distress (reverse sneeze) which was transient in nature and had no effect on the desired action of the drug.
Indications
As an aid in tranquilization and as a preanesthetic agent in dogs.
Acepromazine Maleate Tablets can be used as an aid in controlling intractable animals during examination, treatment, grooming, x-ray and minor surgical procedures.
Contraindications
Phenothiazines may potentiate the toxicity of organophosphates. Therefore, do not use acepromazine maleate to control tremors associated with organic phosphate poisoning.
Do not use in conjunction with organophosphorus vermifuges or ectoparasiticides, including flea collars.
Do not use with procaine hydrochloride.
Cautions
Tranquilizers are potent central nervous system depressants, and they can cause marked sedation with suppression of the sympathetic nervous system.
Tranquilizers can produce prolonged depression or motor restlessness when given in excessive amounts or when given to sensitive animals.
Tranquilizers are additive in action to the actions of other depressants and will potentiate general anesthesia. Tranquilizers should be administered in smaller doses and with greater care during general anesthesia and also to animals exhibiting symptoms of stress, debilitation, cardiac disease, sympathetic blockade, hypovolemia or shock. Acepromazine, like other phenothiazine derivatives, is detoxified in the liver; therefore, it should be used with caution on animals with a previous history of liver dysfunction or leukopenia. Epinephrine is contraindicated for treatment of acute hypotension produced by phenothiazine-derivative tranquilizers since further depression of blood pressure can occur.
Phenothiazines should be used with caution when followed by epidural anesthetic procedures because they may potentiate the arterial hypotensive effects of local anesthetics.
A few rare but serious occurrences of idiosyncratic reactions to Acepromazine may occur in dogs following oral or parenteral administration. These potentially serious adverse reactions include behavioral disorders in dogs such as aggression, biting/chewing, and nervousness.
Dosage and Administration
Dogs: 0.25 - 1.0 mg/lb of body weight. Dosage may be repeated as required.
How Supplied
Acepromazine Maleate Tablets are available in 10 & 25 mg concentrations, and are quarter scored for convenience of administration. Both concentrations are available in bottles of 100 and 500 tablets.
| ACEPROMAZINE MALEATE
acepromazine maleate tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Boehringer Ingelheim Vetmedica, Inc. (007134091) |