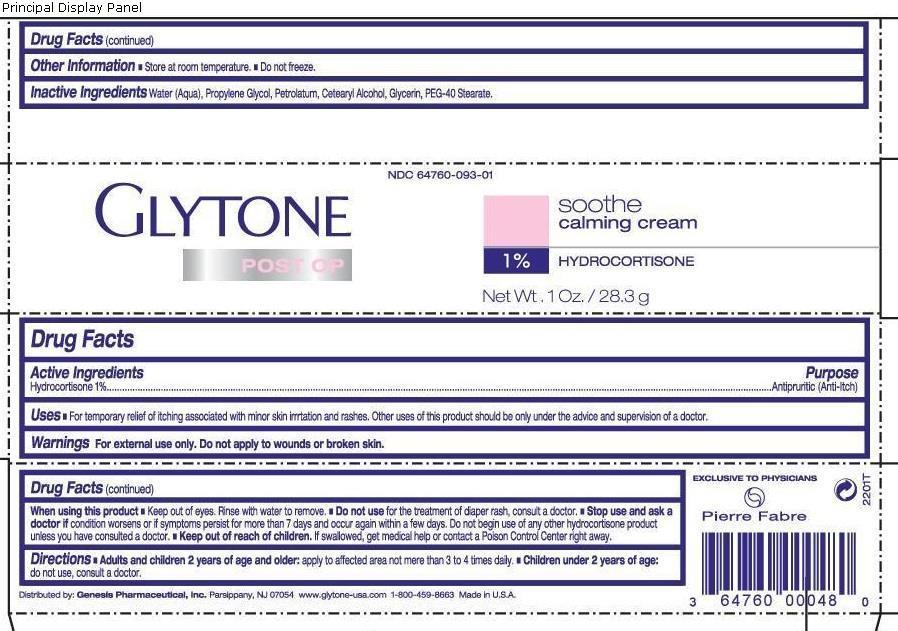
GLYTONE SOOTHE CALMING- hydrocortisone cream
Genesis Pharmaceutical, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Glytone Soothe Calmining
Use(s)
For temporary releief of itching associated with minor irritation and rashes. Other uses of this product should be only under the advice and supervision of a doctor
Warnings
Warnings For external use only. Do not apply to wounds or broken skin.
Directions
- Adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
- Children under 2 years of age: do not use, consult a doctor.
| GLYTONE SOOTHE CALMING
hydrocortisone cream |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Genesis Pharmaceutical, Inc. (117196928) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genesis Pharmaceutical, Inc. | 784338563 | MANUFACTURE(64760-093) | |
Revised: 7/2014
Document Id: 26a7019c-5985-4943-b84f-3478de2c41cf
Set id: 4337762e-8d09-4722-961c-c624e16c1a20
Version: 8
Effective Time: 20140723
Genesis Pharmaceutical, Inc.