BOUCONC. 13-001
- iodine liquid
BouMatic, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
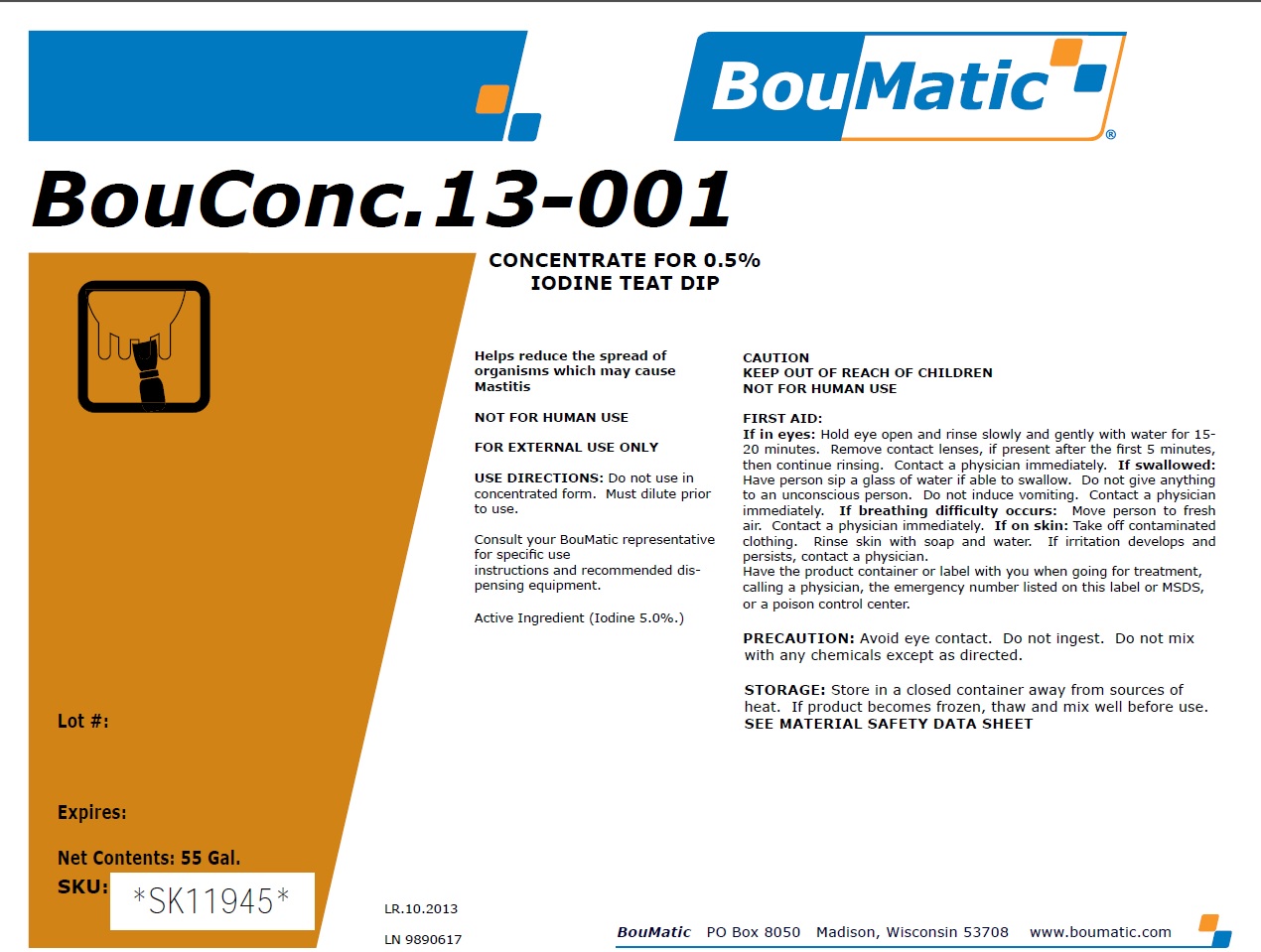
BouConc. 13-001
Concentrate for 0.5% Iodine Teat Dip
Helps reduce the spread of organisms which may cause Mastitis
NOT FOR HUMAN USE
FOR EXTERNAL USE ONLY
USE DIRECTIONS: Do not use in concentrated form. Must
dilute prior to use.
Consult your BouMatic representative for specific use
instructions and recommended dispensing equipment.
Active Ingredient (Iodine 5.0%.)
CAUTION
CAUTION
KEEP OUT OF REACH OF CHILDREN
NOT FOR HUMAN USE
FIRST AID:
If in eyes: Hold eye open and rinse slowly and gently with water for 15-
20 minutes. Remove contact lenses, if present after the first 5 minutes,
then continue rinsing. Contact a physician immediately.
If swallowed: Have person sip a glass of water if able to swallow. Do not give anything
to an unconscious person. Do not induce vomiting. Contact a physician
immediately.
If breathing difficulty occurs: Move person to fresh
air. Contact a physician immediately.
If on skin: Take off contaminated clothing. Rinse skin with soap and water. If irritation develops and persists,
contact a physician.
Have the product container or label with you when going for treatment,
calling a physician, the emergency number listed on this label or MSDS, or
a poison control center.
PRECAUTION: Avoid eye contact. Do not ingest. Do not mix with any chemicals except as directed.
STORAGE: Store in a closed container away from sources of heat. If product becomes frozen, thaw and mix well before use.
SEE MATERIAL SAFETY DATA SHEET
| BOUCONC. 13-001
iodine liquid |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - BouMatic, LLC (124727400) |
| Registrant - BouMatic, LLC (124727400) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BouMatic, LLC | 124727400 | api manufacture, manufacture | |