MAJOR HEARTBURN RELIEF MAXIMUM STRENGTH
-
famotidine tablet
Major Pharmaceuticals
----------
Major Pharmaceuticals Heartburn Relief Drug FactsActive ingredient (in each tablet)
Famotidine 20 mg
Purpose
Acid reducer
Uses
- relieves heartburn associated with acid indigestion and sour stomach
- prevents heartburn associated with acid indigestion and sour stomach brought on by eating or drinking certain food and beverages
Warnings
Allergy alert: Do not use if you are allergic to famotidine or other acid reducers
Do not use
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
- if you have kidney disease, except under the advice and supervision of a doctor
- with other acid reducers
Ask a doctor before use if you have
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating, or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
Stop use and ask a doctor if
- your heartburn continues or worsens
- you need to take this product for more than 14 days
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 12 years and over:
- to relieve symptoms, swallow 1 tablet with a glass of water. Do not chew.
- to prevent symptoms, swallow 1 tablet with a glass of water at any time from 10 to 60 minutes before eating food or drinking beverages that cause heartburn
- do not use more than 2 tablets in 24 hours
- children under 12 years: ask a doctor
Other information
- read the directions and warnings before use
- keep the carton. It contains important information.
- store at 20°-25°C (68°-77°F)
- protect from moisture and light
Inactive ingredients
carnauba wax, colloidal silicon dioxide, croscarmellose sodium, lactose (monohydrate), magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide
Questions or comments?
1-800-719-9260
Principal Display Panel
Maximum Strength
Heartburn Relief
Famotidine Tablets, 20 mg
Acid Reducer
Just One Tablet
Prevents & Relieves Heartburn Due to Acid Indigestion
Compare to the active ingredient of Maximum Strength Pepcid® AC
Actual Size
Tablets
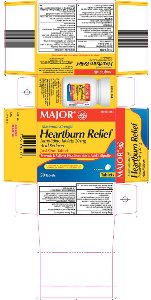
Heartburn Relief Carton
| MAJOR HEARTBURN RELIEF
MAXIMUM STRENGTH
famotidine tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA077351 | 09/29/2006 | |
| Labeler - Major Pharmaceuticals (191427277) |