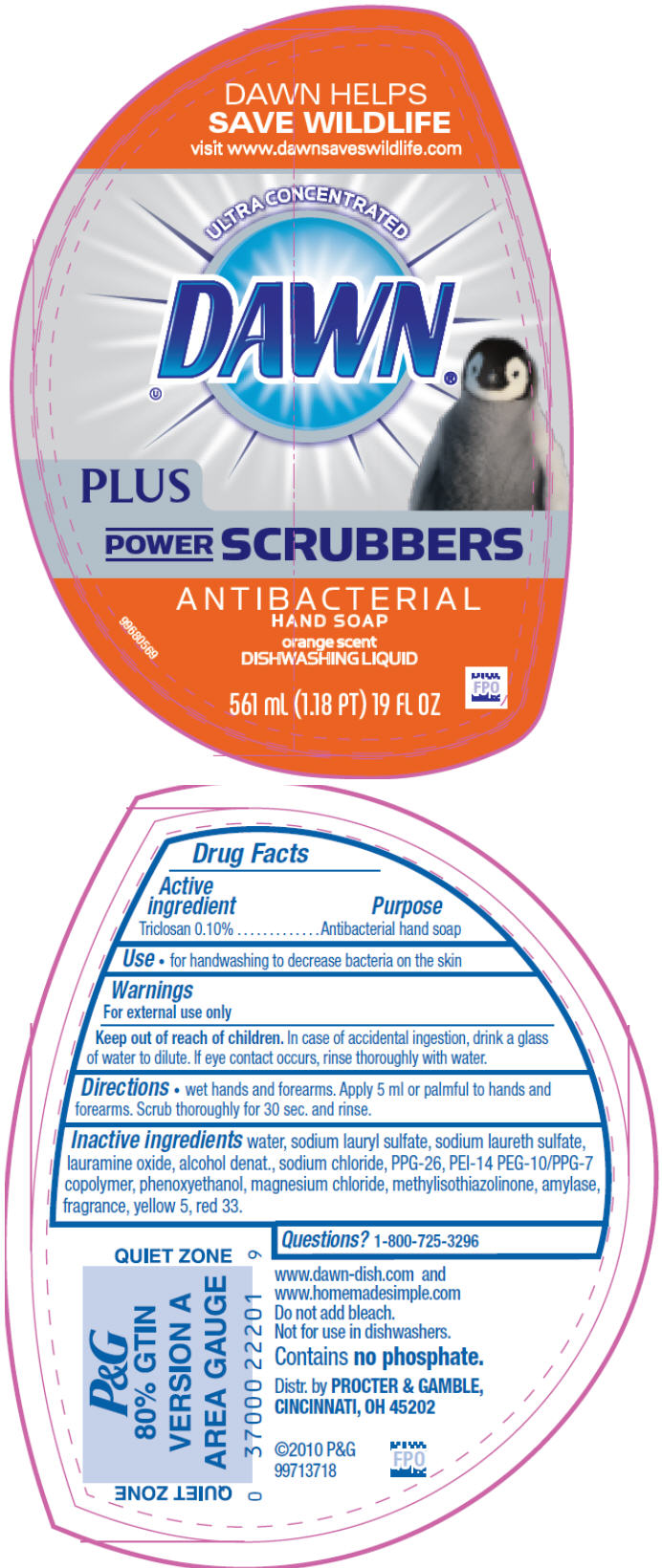
DAWN ULTRA CONCENTRATED POWER SCRUBBER ORANGE- triclosan soap
Procter & Gamble Manufacturing Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Dawn Ultra Concentrated plus Power Scrubber Orange AB
Directions
- Wet hands and forearms. Apply 5 ml or palmful to hands and forearms. Scrub thoroughly for 30 sec. and rinse
| DAWN
ULTRA CONCENTRATED POWER SCRUBBER ORANGE
triclosan soap |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Procter & Gamble Manufacturing Company (004238200) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| The Procter & Gamble Manufacturing Company | 007130032 | ANALYSIS(37000-612), LABEL(37000-612), MANUFACTURE(37000-612), PACK(37000-612), RELABEL(37000-612), REPACK(37000-612) | |
Revised: 6/2014
Document Id: 67ee9d47-a057-4417-ae8b-bf5a6c7efef9
Set id: f99cb853-8fcc-4f4b-bf2a-9ce2e5709ffb
Version: 2
Effective Time: 20140610
Procter & Gamble Manufacturing Company